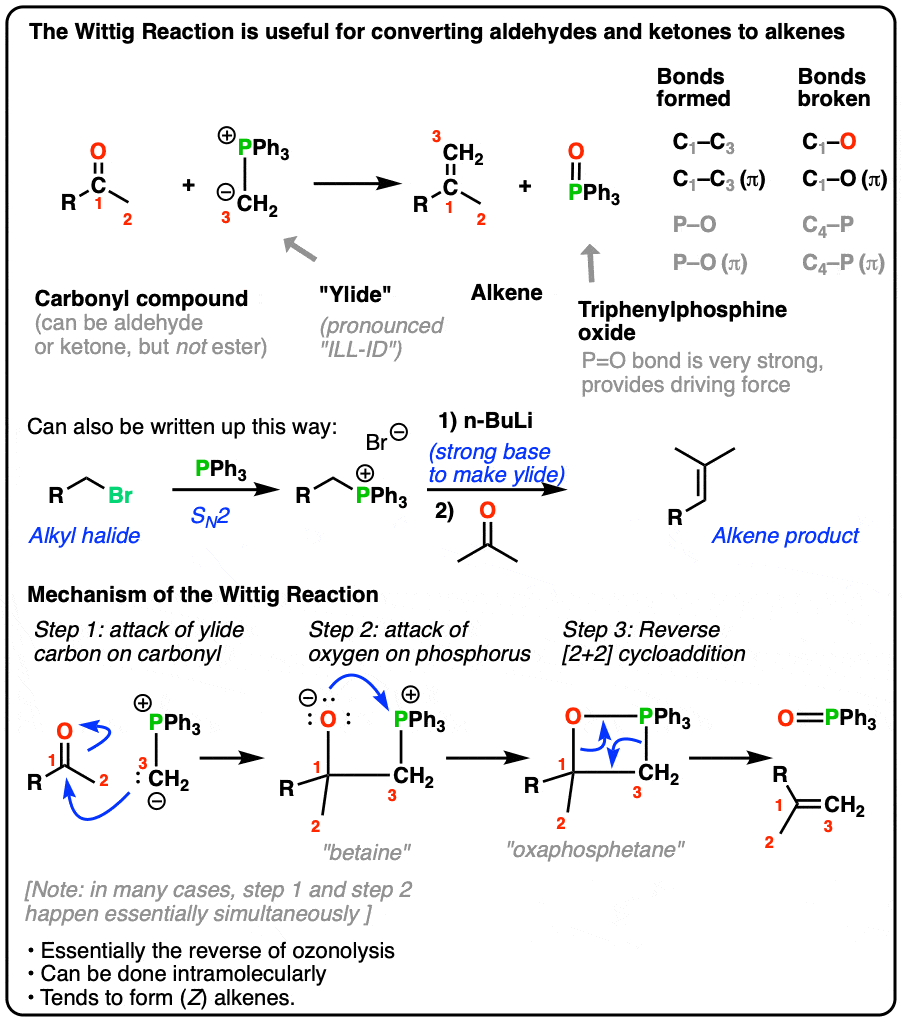
Synthesis of Unsymmetrical Bis(phosphine) Oxides and Their Phosphines via Secondary Phosphine Oxide Precursors | SpringerLink

Secondary phosphine oxides: Versatile ligands in transition metal-catalyzed cross-coupling reactions - ScienceDirect
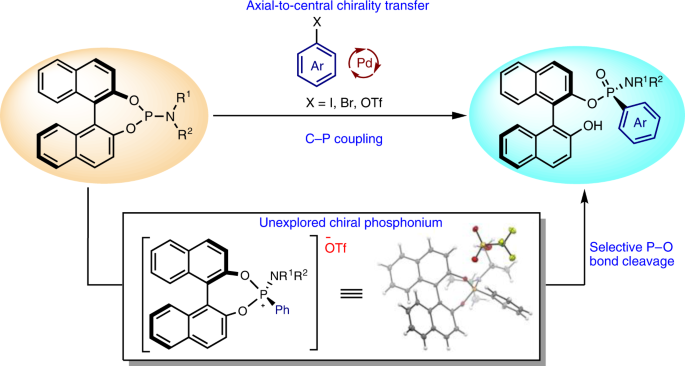
P-chirogenic phosphorus compounds by stereoselective Pd-catalysed arylation of phosphoramidites | Nature Catalysis
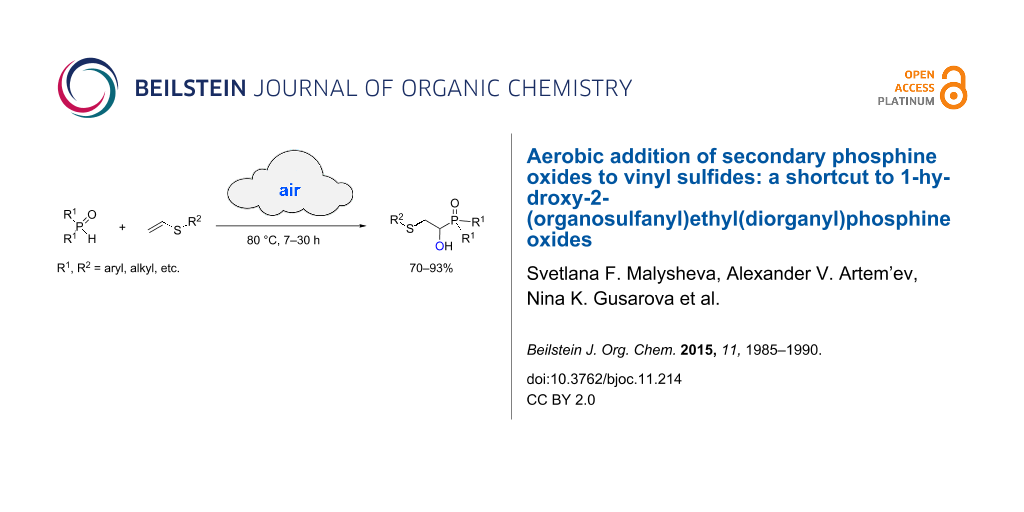
BJOC - Aerobic addition of secondary phosphine oxides to vinyl sulfides: a shortcut to 1-hydroxy-2-(organosulfanyl)ethyl(diorganyl)phosphine oxides

Mechanistic aspects of the stereospecific reduction of chiral hydroxyalkyl phosphinates and phosphine oxides
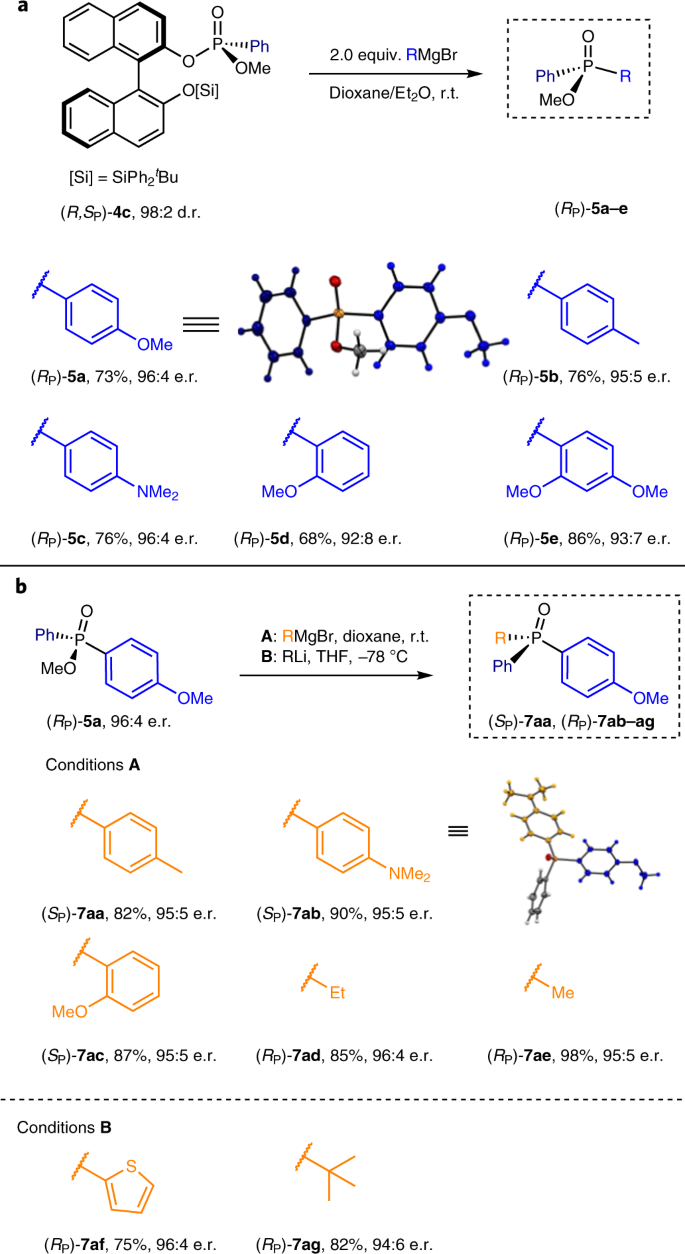
P-chirogenic phosphorus compounds by stereoselective Pd-catalysed arylation of phosphoramidites | Nature Catalysis

Catalytic Asymmetric Synthesis of Phosphine Boronates - Hornillos - 2015 - Angewandte Chemie International Edition - Wiley Online Library

Secondary phosphine oxides: Versatile ligands in transition metal-catalyzed cross-coupling reactions - ScienceDirect

Enantioselective Cu-Catalyzed Arylation of Secondary Phosphine Oxides with Diaryliodonium Salts toward the Synthesis of P-Chiral Phosphines | Semantic Scholar

PDF) Conversion of triphenylphosphine oxide to organophosphorus via selective cleavage of C-P, O-P, and C-H bonds with sodium

Secondary phosphine oxides: Versatile ligands in transition metal-catalyzed cross-coupling reactions - ScienceDirect
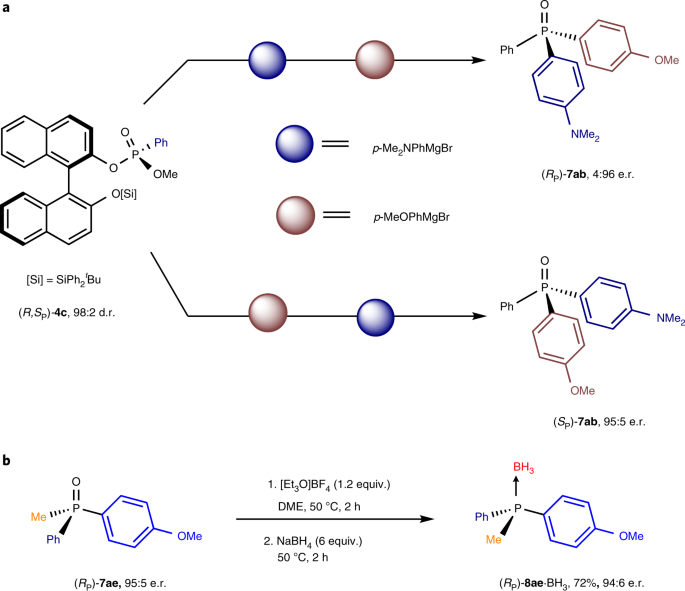
P-chirogenic phosphorus compounds by stereoselective Pd-catalysed arylation of phosphoramidites | Nature Catalysis
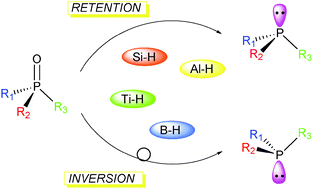
Reduction of secondary and tertiary phosphine oxides to phosphines - Chemical Society Reviews (RSC Publishing)

C–F Activation for C(sp2)–C(sp3) Cross-Coupling by a Secondary Phosphine Oxide (SPO)-Nickel Complex | Organic Letters

Ni-Catalyzed Asymmetric Allylation of Secondary Phosphine Oxides | Journal of the American Chemical Society

Advancing Air‐ and Moisture‐Compatible s‐Block Organometallic Chemistry Using Sustainable Solvents - García‐Garrido - 2021 - European Journal of Inorganic Chemistry - Wiley Online Library

Synthesis of Unsymmetrical Tertiary Phosphine Oxides via Sequential Substitution Reaction of Phosphonic Acid Dithioesters with Grignard Reagents | Organic Letters

Mechanistic aspects of the stereospecific reduction of chiral hydroxyalkyl phosphinates and phosphine oxides

Facial conversion of secondary phosphine oxides R1R2P(O)H to chlorophosphines R1R2PCl by acetyl chloride - ScienceDirect

Enantioselective Cu-Catalyzed Arylation of Secondary Phosphine Oxides with Diaryliodonium Salts toward the Synthesis of P-Chiral Phosphines | Semantic Scholar