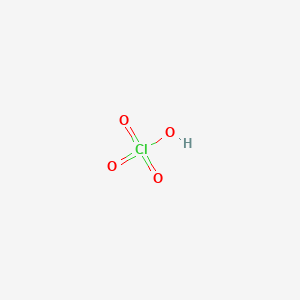
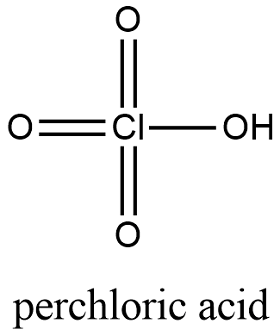
![7601-90-3・Perchloric Acid・166-00713・162-00715[Detail Information] | [Common Chemicals & Lab Tools]|Laboratory Chemicals-FUJIFILM Wako Chemicals Europe GmbH 7601-90-3・Perchloric Acid・166-00713・162-00715[Detail Information] | [Common Chemicals & Lab Tools]|Laboratory Chemicals-FUJIFILM Wako Chemicals Europe GmbH](https://labchem-wako.fujifilm.com/sc/05/162-00715.png)
7601-90-3・Perchloric Acid・166-00713・162-00715[Detail Information] | [Common Chemicals & Lab Tools]|Laboratory Chemicals-FUJIFILM Wako Chemicals Europe GmbH

SOLVED: A concentrated perchloric acid solution is 72.296 HCIO4 by mass and its density is 1.68 g mL - What volume (In litres) of the concentrated perchloric acid solution Is needed to
![PDF] Heats of formation of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. Probing the limits of W1 and W2 theory | Semantic Scholar PDF] Heats of formation of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. Probing the limits of W1 and W2 theory | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6f8d5720d3ec745b4442ba99027bfaae699e2eab/17-TableI-1.png)
PDF] Heats of formation of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. Probing the limits of W1 and W2 theory | Semantic Scholar
![PDF] Anharmonic force fields of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. An extreme case of inner polarization | Semantic Scholar PDF] Anharmonic force fields of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. An extreme case of inner polarization | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7387cef1aaa9bac26d23cbc60d37f3d7a379d06c/13-TableI-1.png)
PDF] Anharmonic force fields of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. An extreme case of inner polarization | Semantic Scholar

SOLVED: Commercial perchloric acid is an aqueous solution containing 70% by mass HCIOs (100.46 g mol-') and has = density of 1.664 gmL-! How many mL of commercial perchloric acid should be
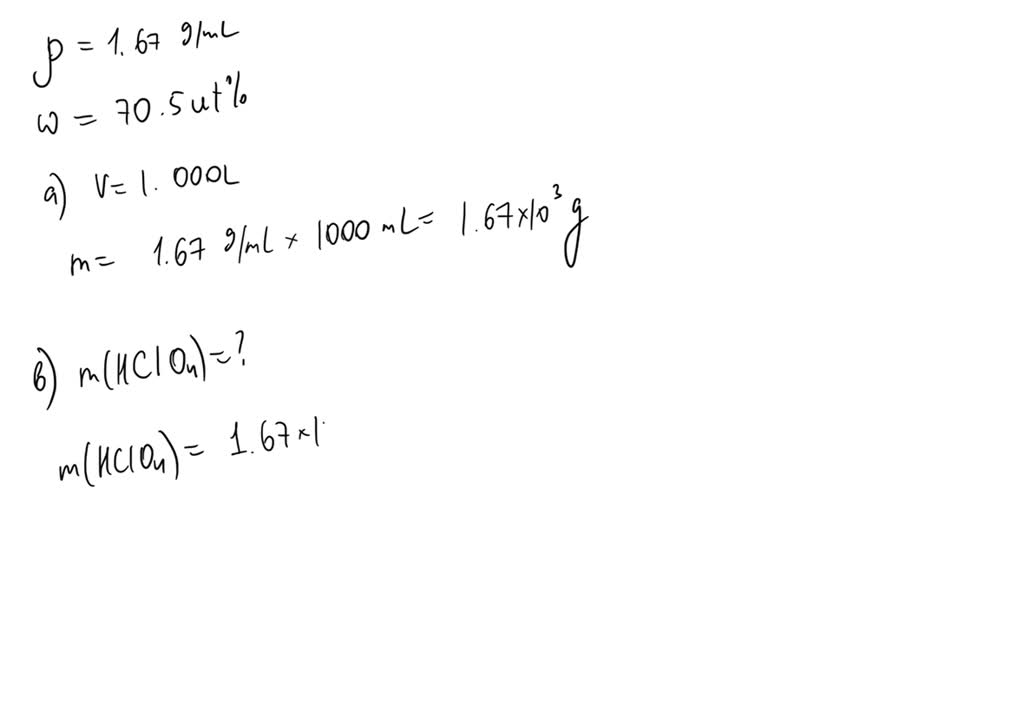
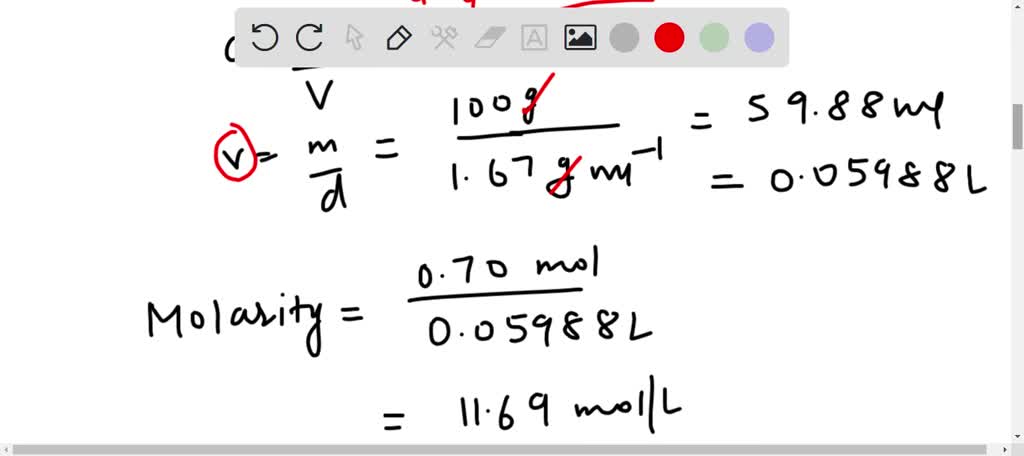
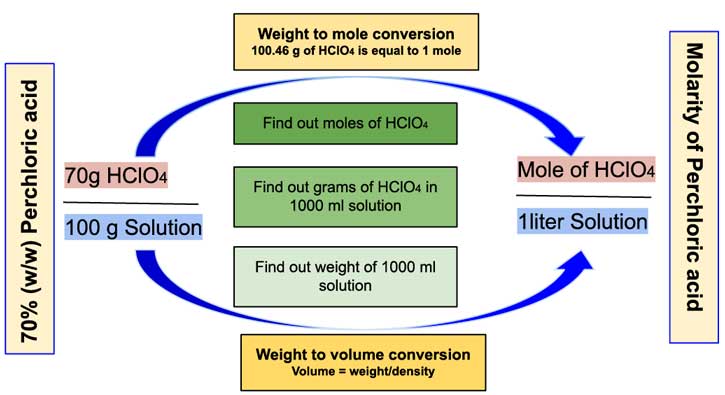
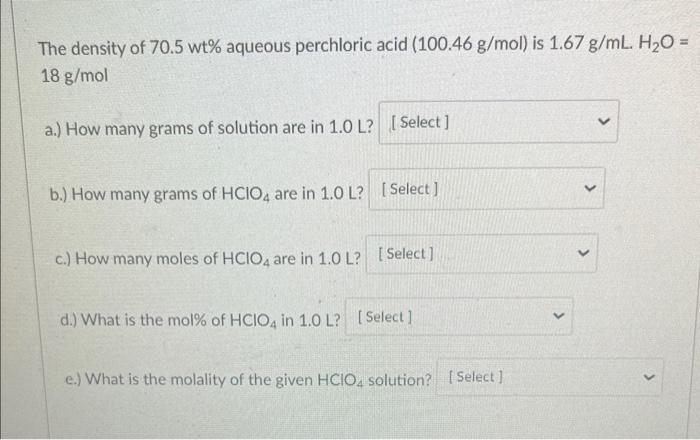
SOLVED: The density of 70.5 wt% aqueous perchloric acid is 1.67 g/mL. Recall that grams refer to grams of solution (5 g HClO4 1 g H2O). (a) How many grams of solution
![PDF] Anharmonic force fields of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. An extreme case of inner polarization | Semantic Scholar PDF] Anharmonic force fields of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. An extreme case of inner polarization | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7387cef1aaa9bac26d23cbc60d37f3d7a379d06c/16-TableIV-1.png)
PDF] Anharmonic force fields of perchloric acid, HClO4, and perchloric anhydride, Cl2O7. An extreme case of inner polarization | Semantic Scholar

SOLVED: A concentrated perchloric acid solution is 68.49 HCIOa by mass and its density is .68 g mL What volume (in litres) of the concentrated perchloric acid solution is needed to make