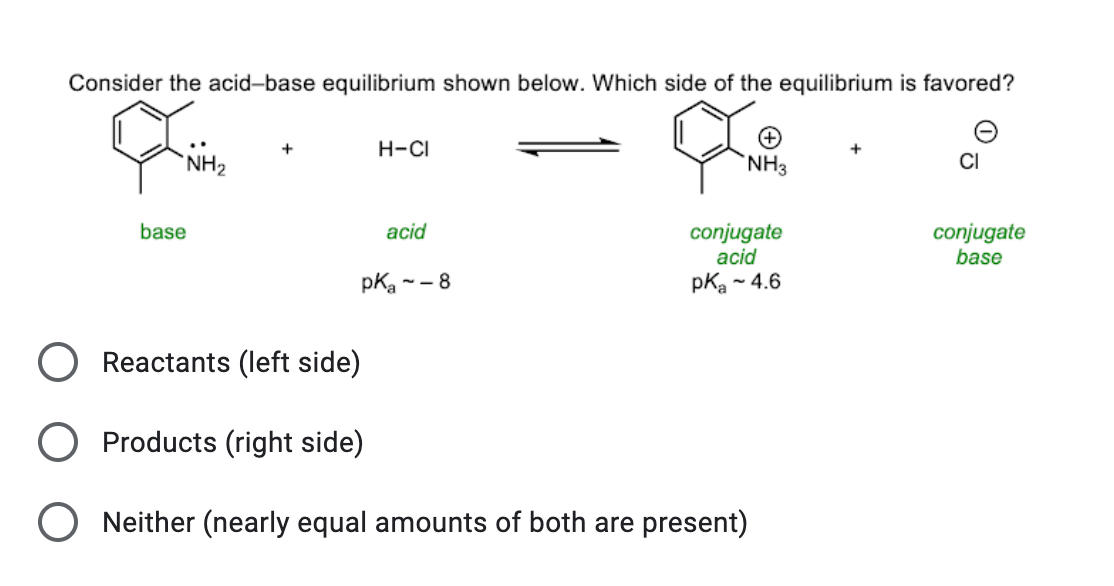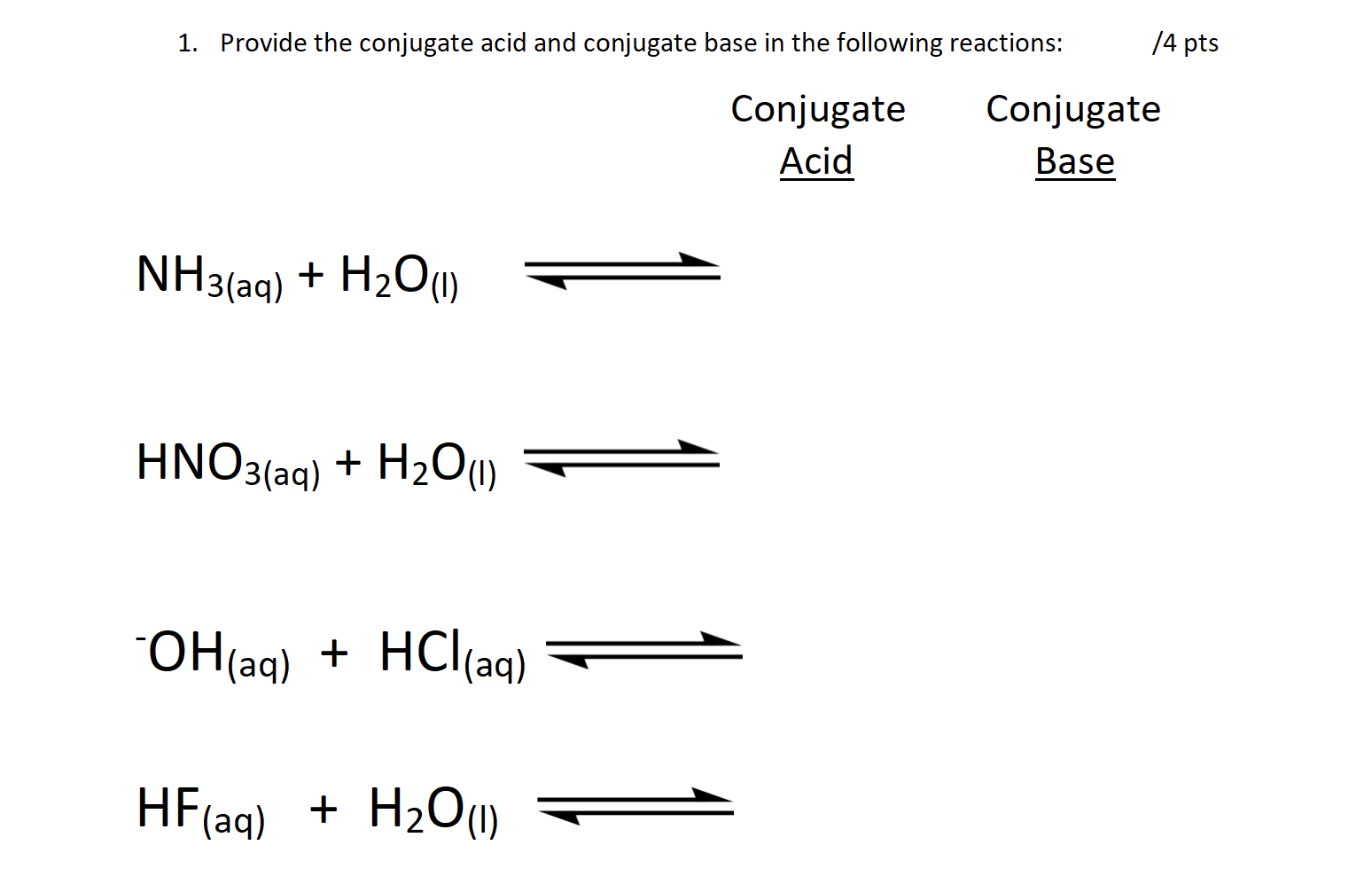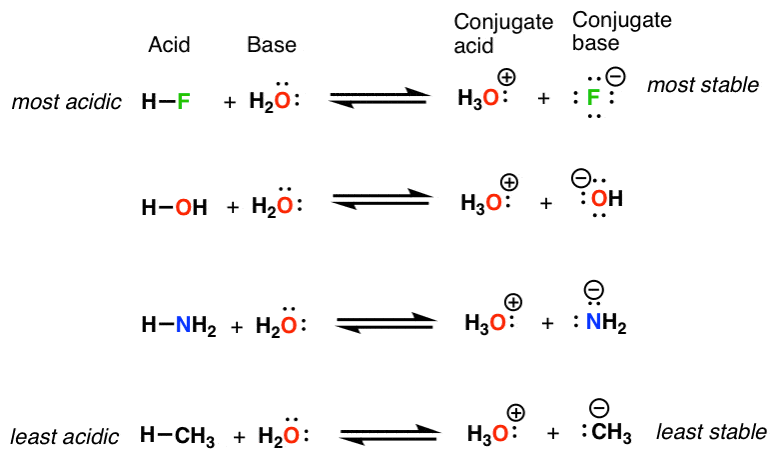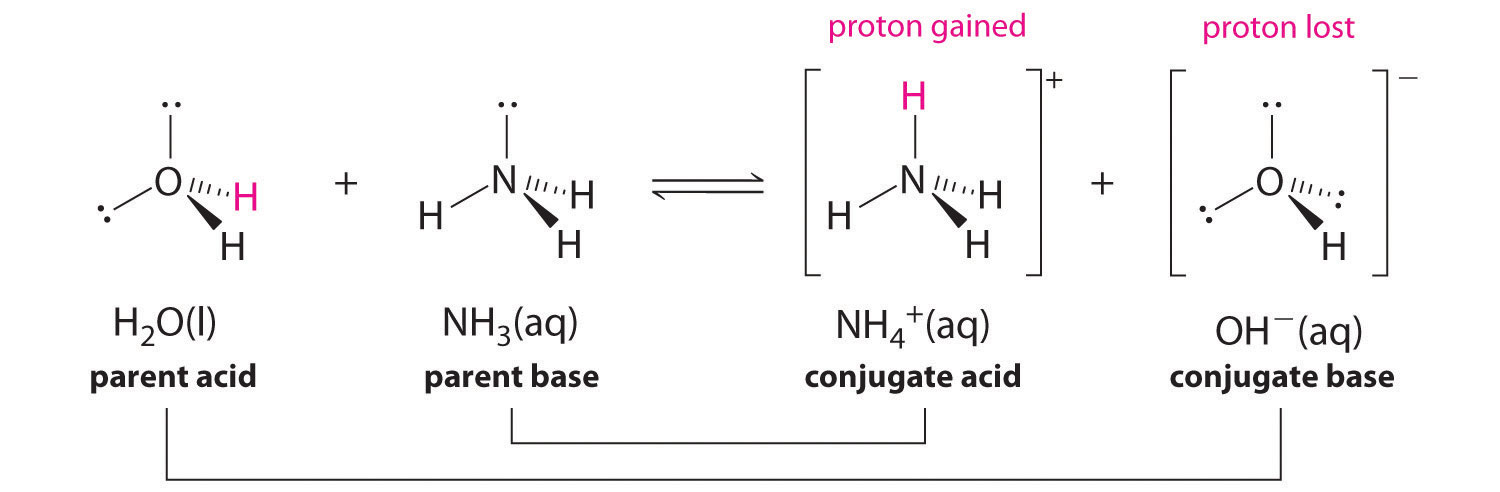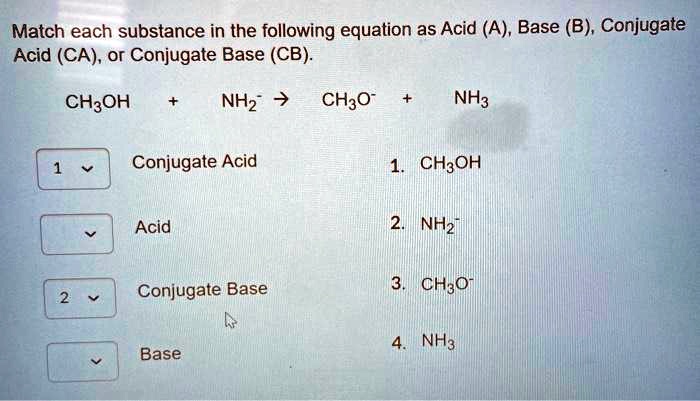
SOLVED: Match each substance in the following equation as Acid (A), Base (B), Conjugate Acid (CA), or Conjugate Base (CB) CH3OH NH2 CH3O" NH3 Conjugate Acid CHzOH Acid NH2 Conjugate Base CH3o-

organic chemistry - Why In This Reaction Acetic Acid is strong acid and NH3 is strong base ?please explain in details and thanks for answer - Chemistry Stack Exchange

NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in
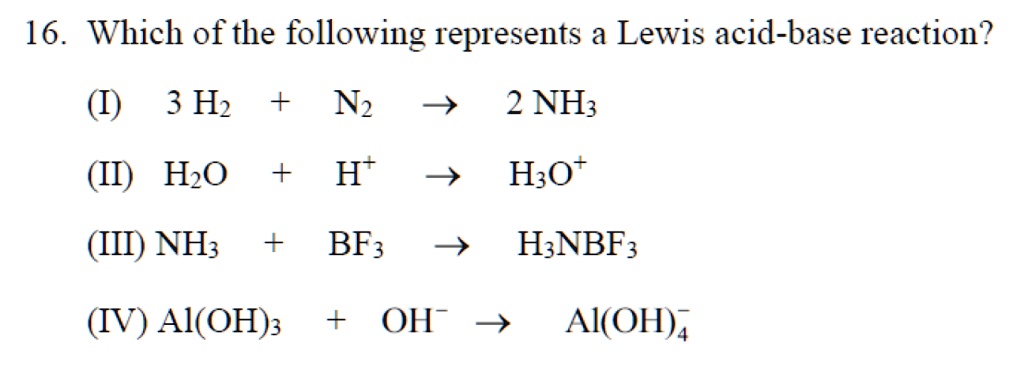
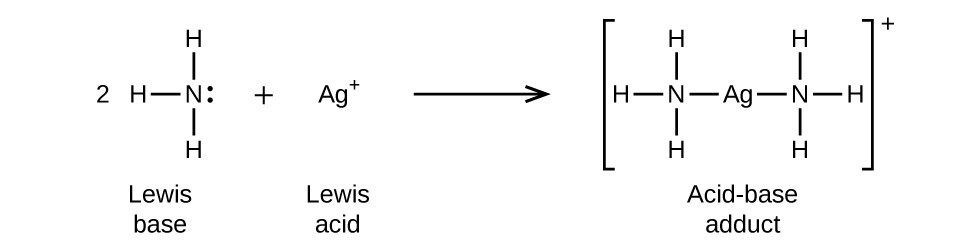
SOLVED: 16. Which of the following represents a Lewis acid-base reaction? (I) 3 Hz + N2 2 NH3 (II) Hzo + H H;ot (III) NH; 1 BF3 HNBF; (IV) AI(OH)3 T OH- AI(OH)A