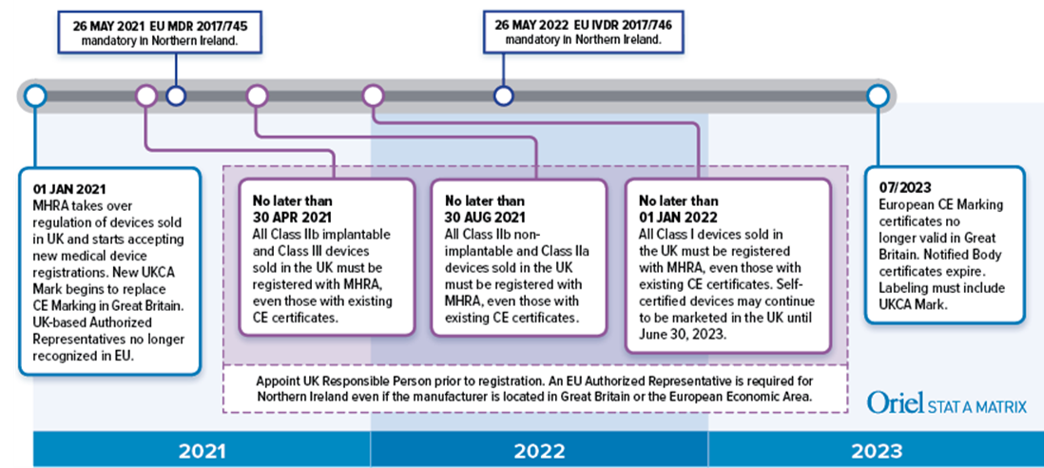
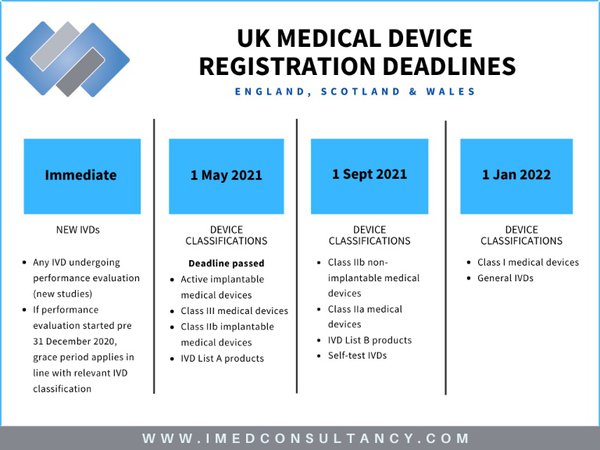
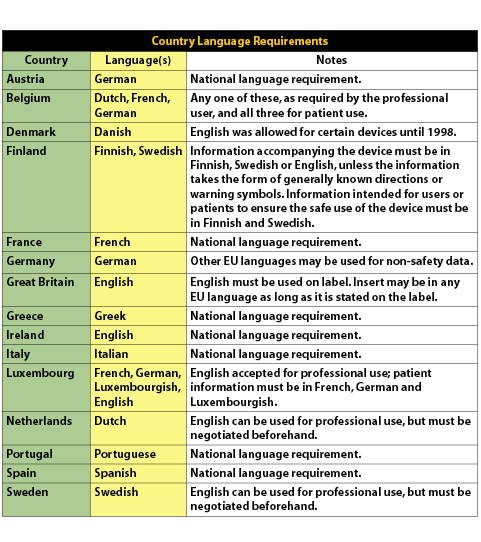
New UK medical device regulation spells potential trial concerns for some IVD players - Medical Device Network

Top 10 Questions regarding the UK Responsible Person and medical device/IVD registration with the MHRA

A Strategic Technology Roadmap for the UK In Vitro Diagnostics Industry – Cambridge Design Partnership