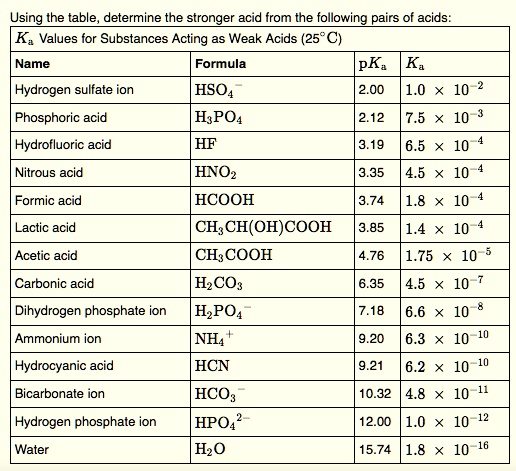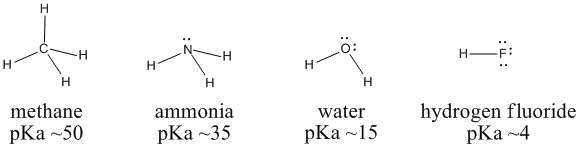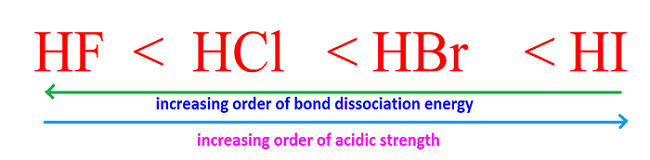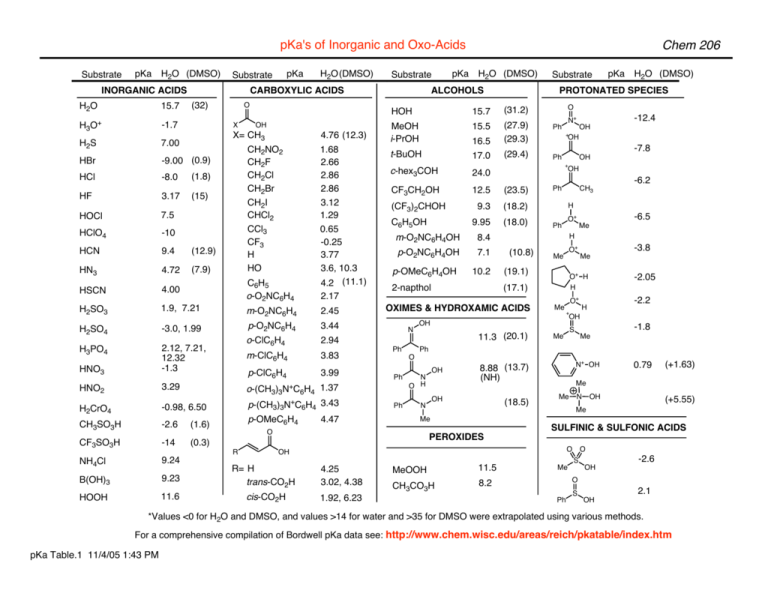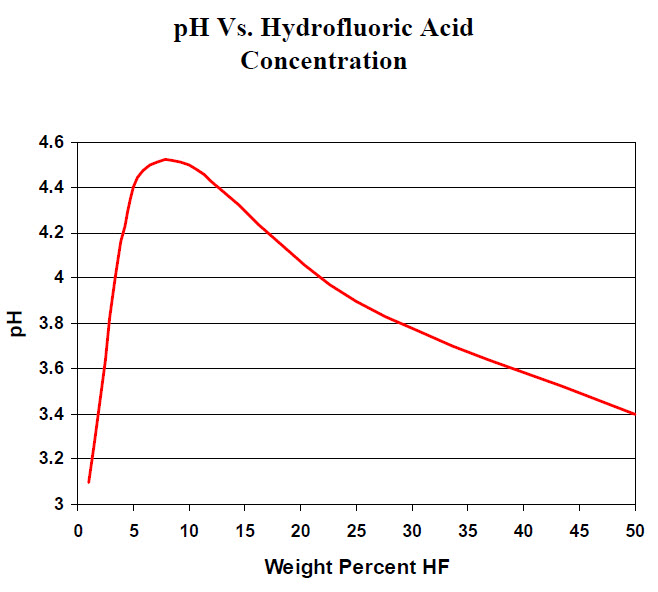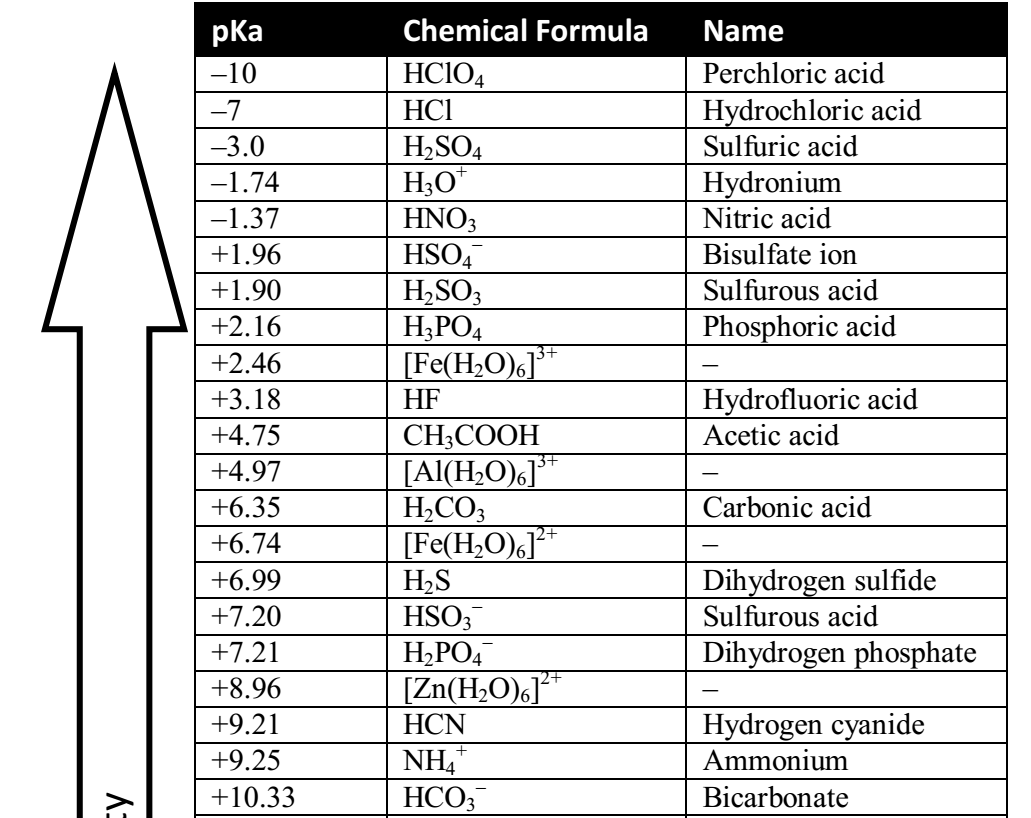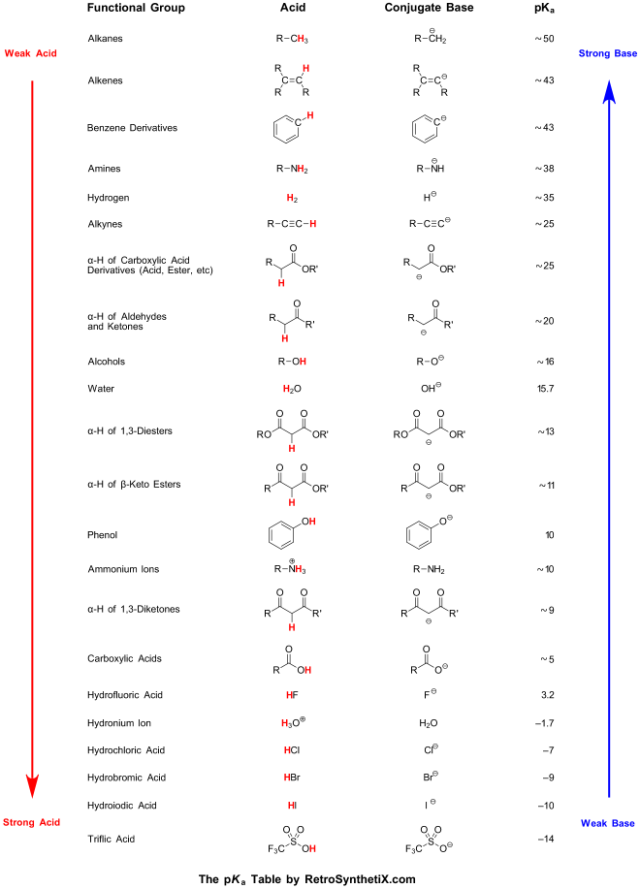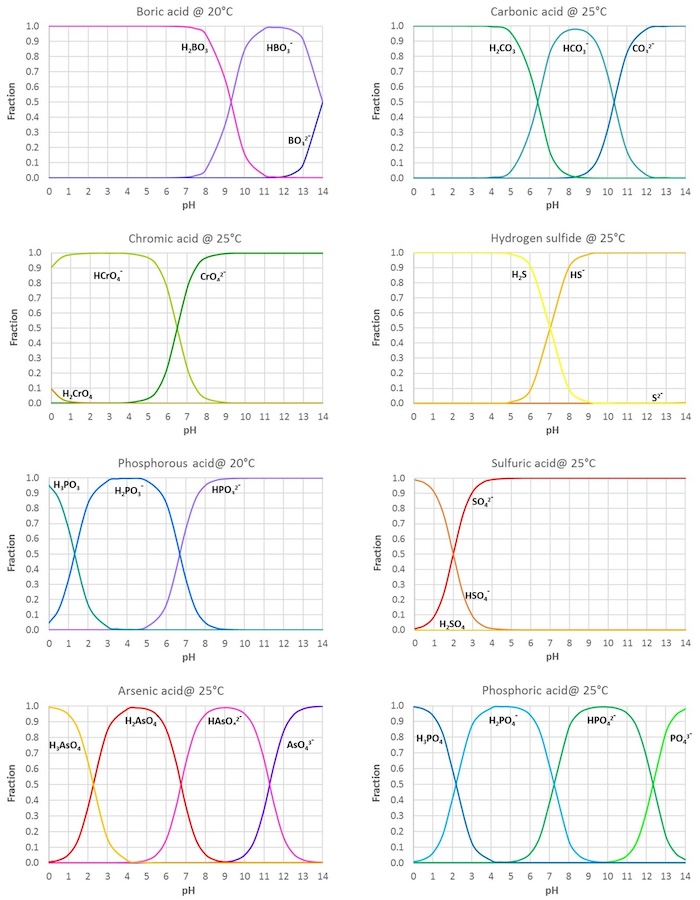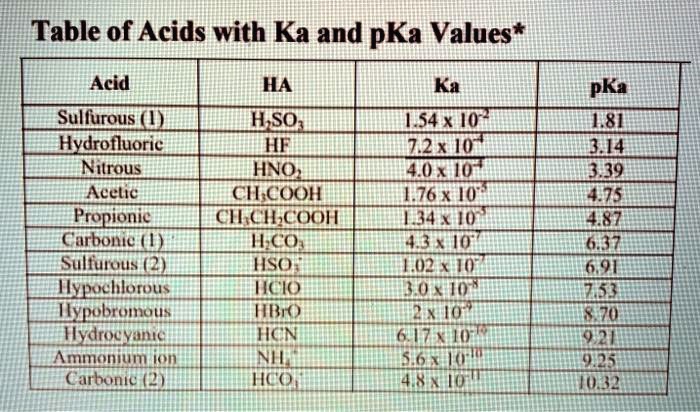
SOLVED: Table of Acids with Ka and pKa Values* Acid HA Ka Sullurous ( 4) HSO; LS4X Ho? Hydrofluoric HF Z2X0I Mutrous HNO; 40Xl0i Aeelic CICOOH L7ox I0 Prepionie CLCLCOOH LL4O Cubonie ()
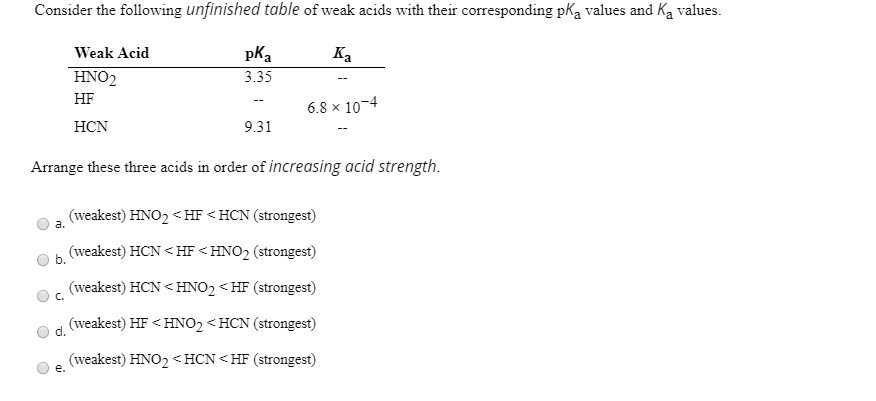
SOLVED: Consider the following unfinished table of weak acids with their corresponding pKa values and Ka values. Weak Acid HNO2 HF pKa 335 6.8 * 10 + HCN 9.31 Arrange these three
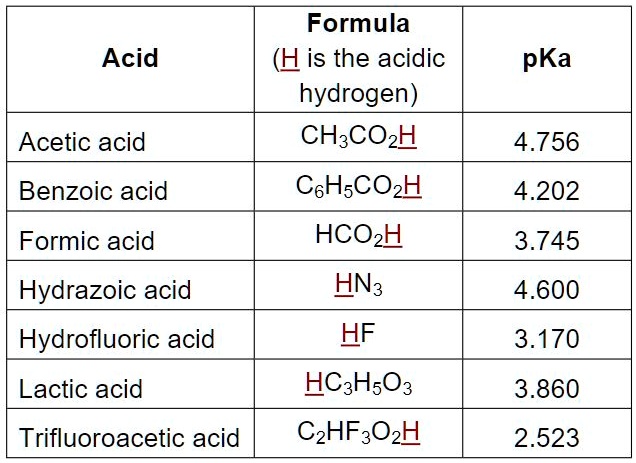
SOLVED: Formula Acid (His the acidic hydrogen) CH:COzH pKa Acetic acid 4.756 Benzoic acid CsHsCOzH HCOzH HN3 HF 4.202 Formic acid 3.745 Hydrazoic acid Hydrofluoric acid Lactic acid 4.600 3.170 HCHsO3 3.860 Trifluoroacetic acid CzHF3OzH 2.523