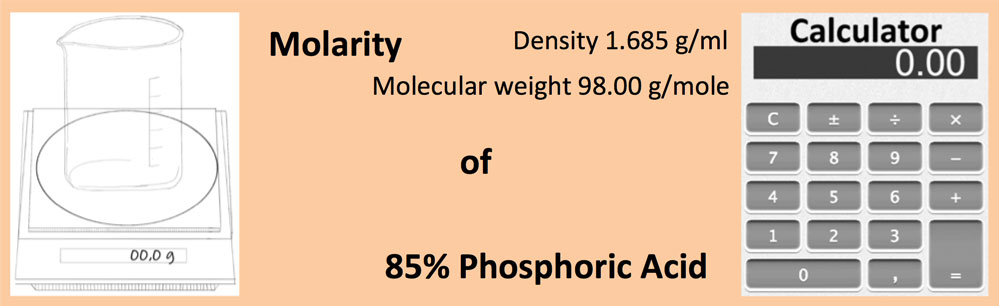
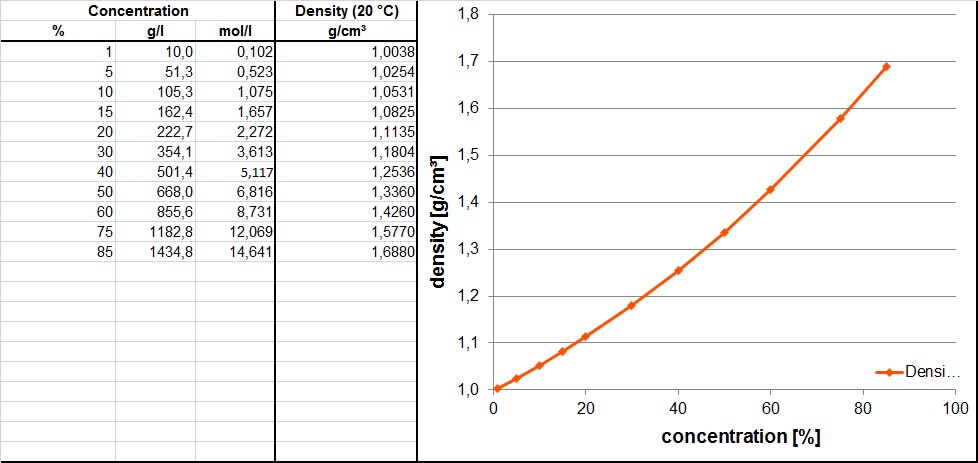
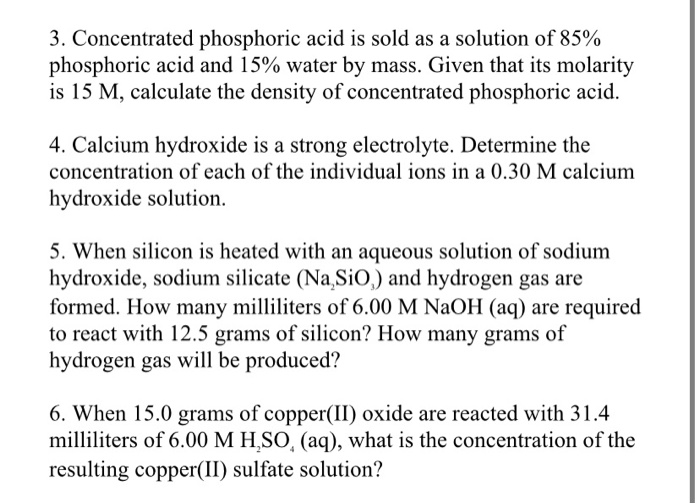
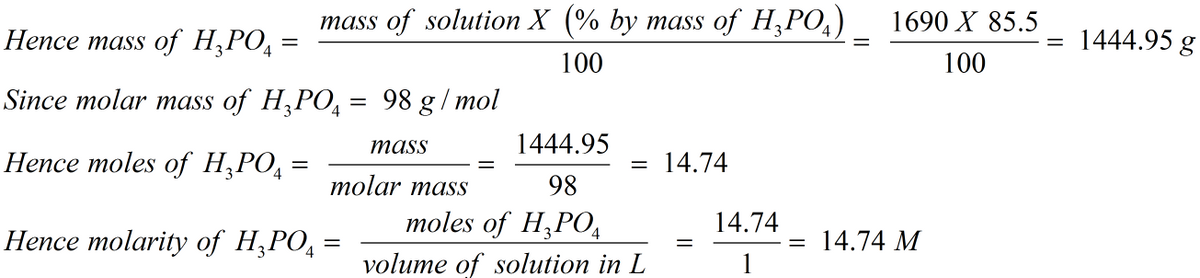If a 15.00 mL sample of phosphoric acid required 13.9 mL of 0.106 M potassium hydroxide to completely neutralize, what is the molarity (mol / L) of the phosphoric acid to the

The density of 85% Phosphoric acid is 1.70 g cm^-3 . What is the volume of a solution that contains 17 gm Phosphoric acid?

Influence of phosphoric acid concentration in the mixture on product pH. | Download Scientific Diagram
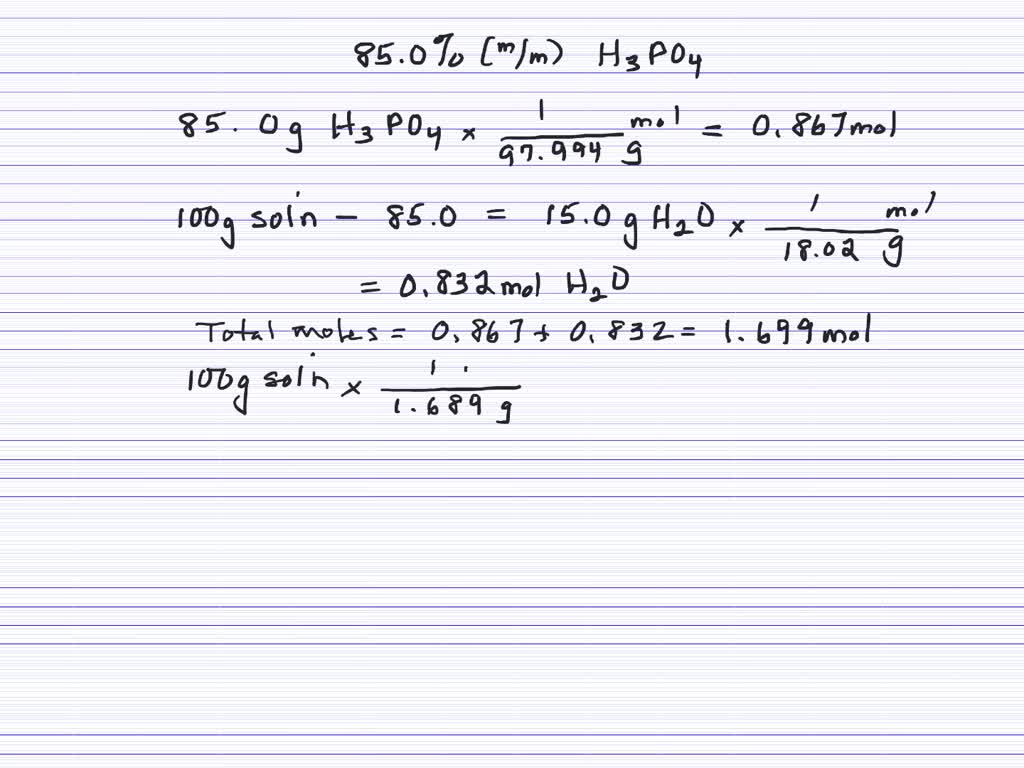
SOLVED:A bottle of phosphoric acid is labeled “ 85.0 % H3 PO4 by mass; density =1.689 g / cm^3 . " Calculate the molarity, molality and mole fraction of the phosphoric acid in solution.
How will you prepare 100ml of 6m orthophosphoric acid mol weight =98; specific gravity=1.75minimum assay-85%? - Quora

SOLVED: A concentrated phosphoric acid solution is 85.5% H3PO4 by mass and has a density of 1.69 g/mL at 25°C. What is the molarity of H3PO4? What is the mole fraction of

SOLVED: A solution of phosphoric acid was made by dissolving 10.1 g of H3PO4 in 136.00 mL of water. The resulting volume was 140. mL. Calculate the density, mole fraction, molarity, and