Biological buffering of blood There are three major contributors to regulating the pH of blood. Bicarbonate, phosphate and proteins Blood pH Must be Kept. - ppt download

The comparison of pKa determination between carbonic acid and formic acid and its application to prediction of the hydration numbers - ScienceDirect

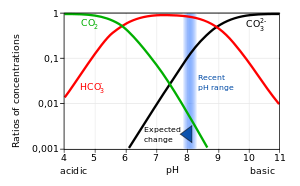
physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange
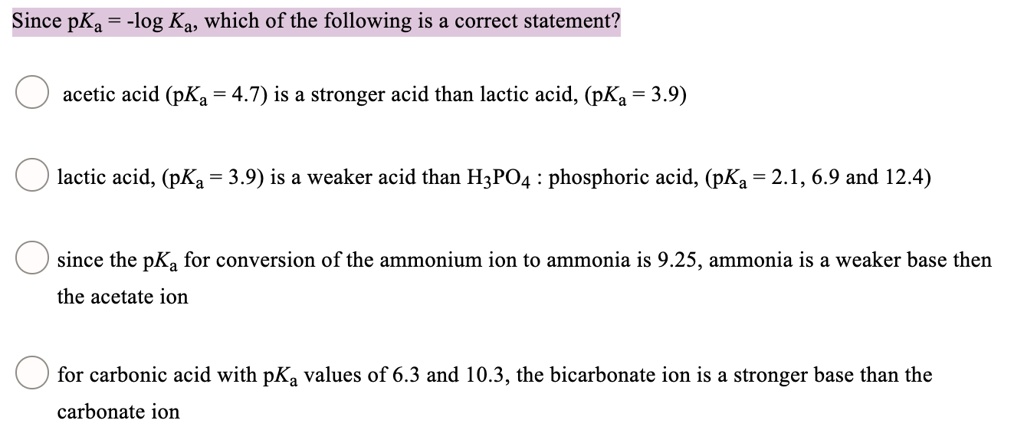
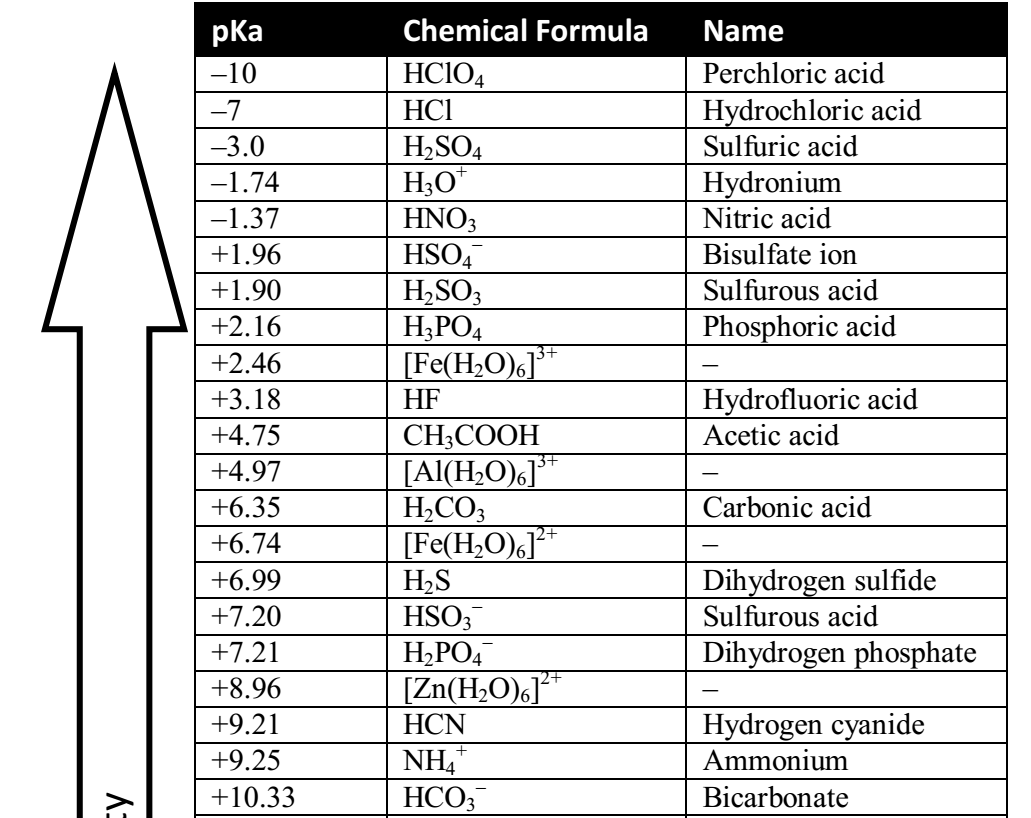
SOLVED: Since pKa -log Ka; which of the following is a correct statement? acetic acid (pKa = 4.7) is a stronger acid than lactic acid, (pKa 3.9) lactic acid, (pKa = 3.9)
Why, when CO2 is accumulated in our body, does it turn to H2CO3, and then dissociate to HCO3- and H+ (? Why does this acid dissociate and increase H+, for no purpose,
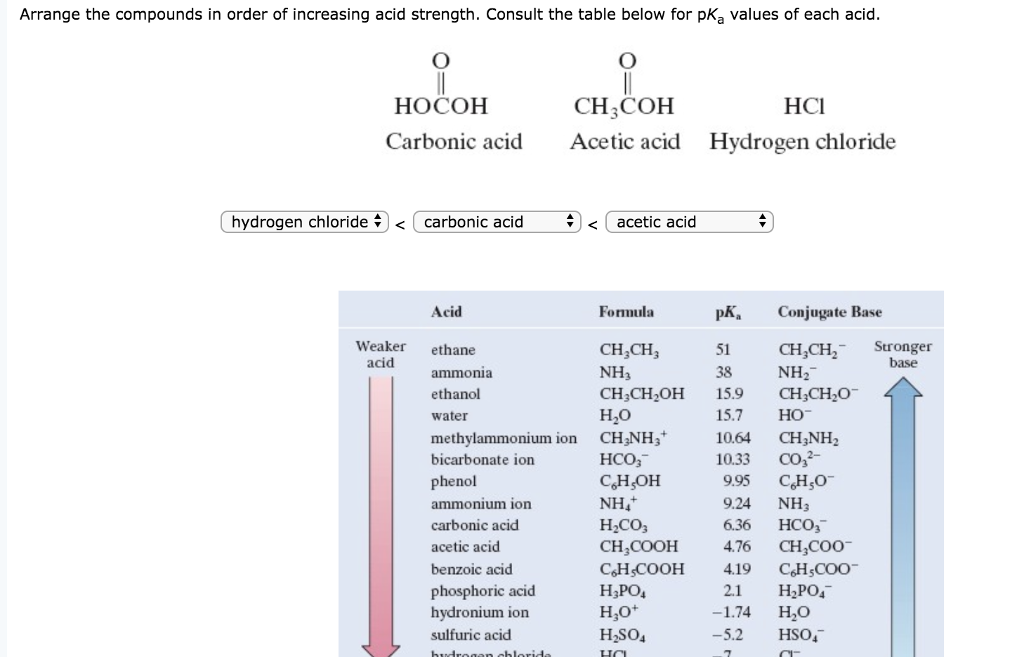
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora
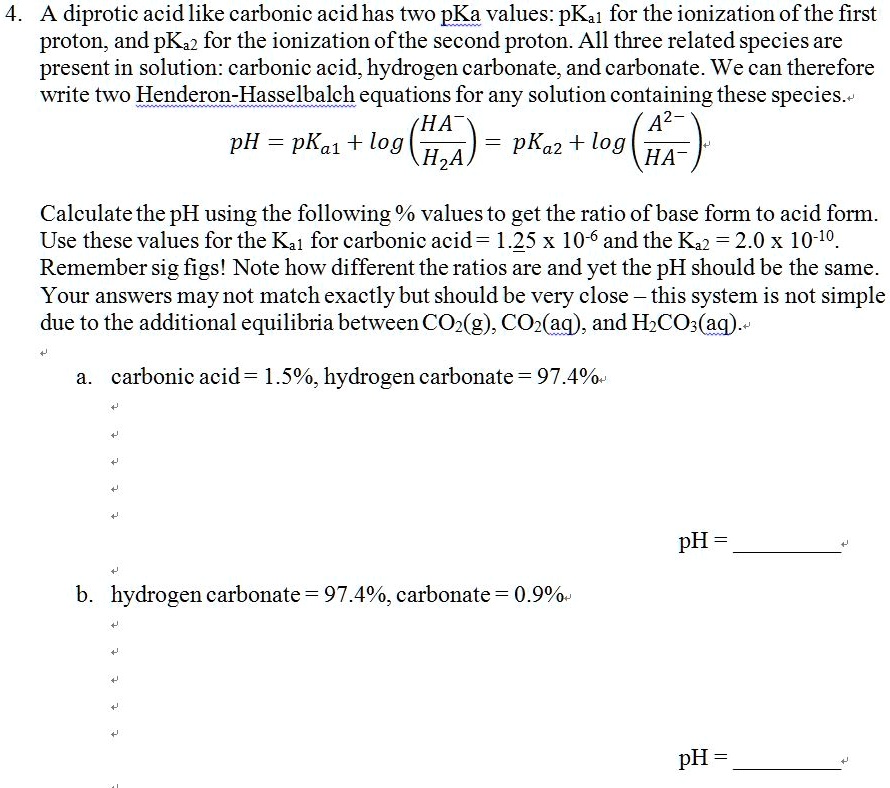
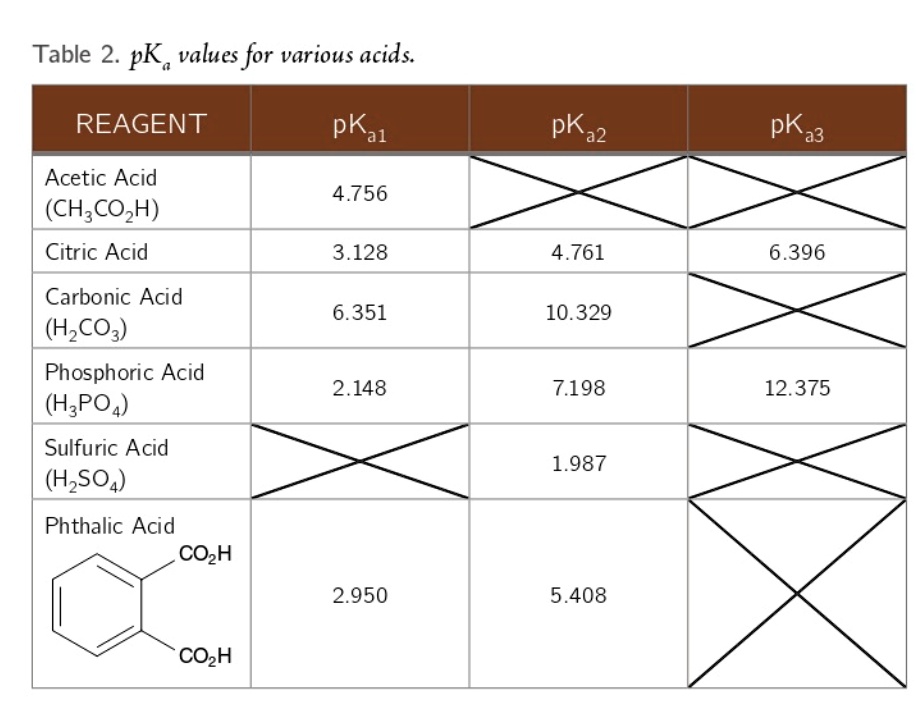
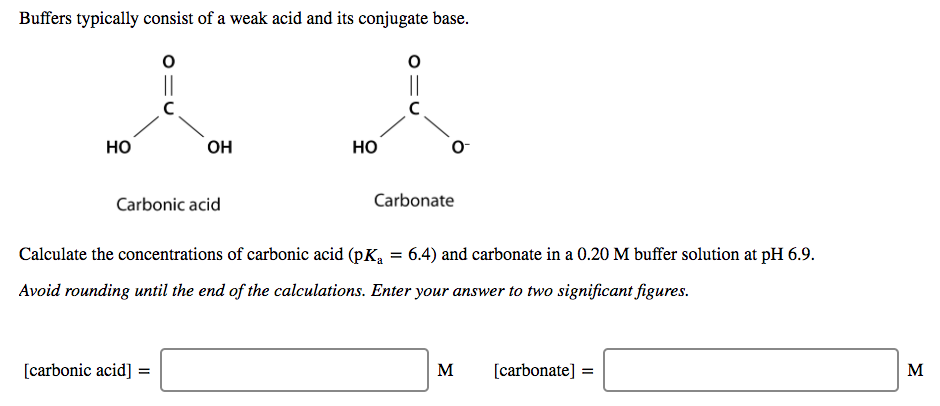
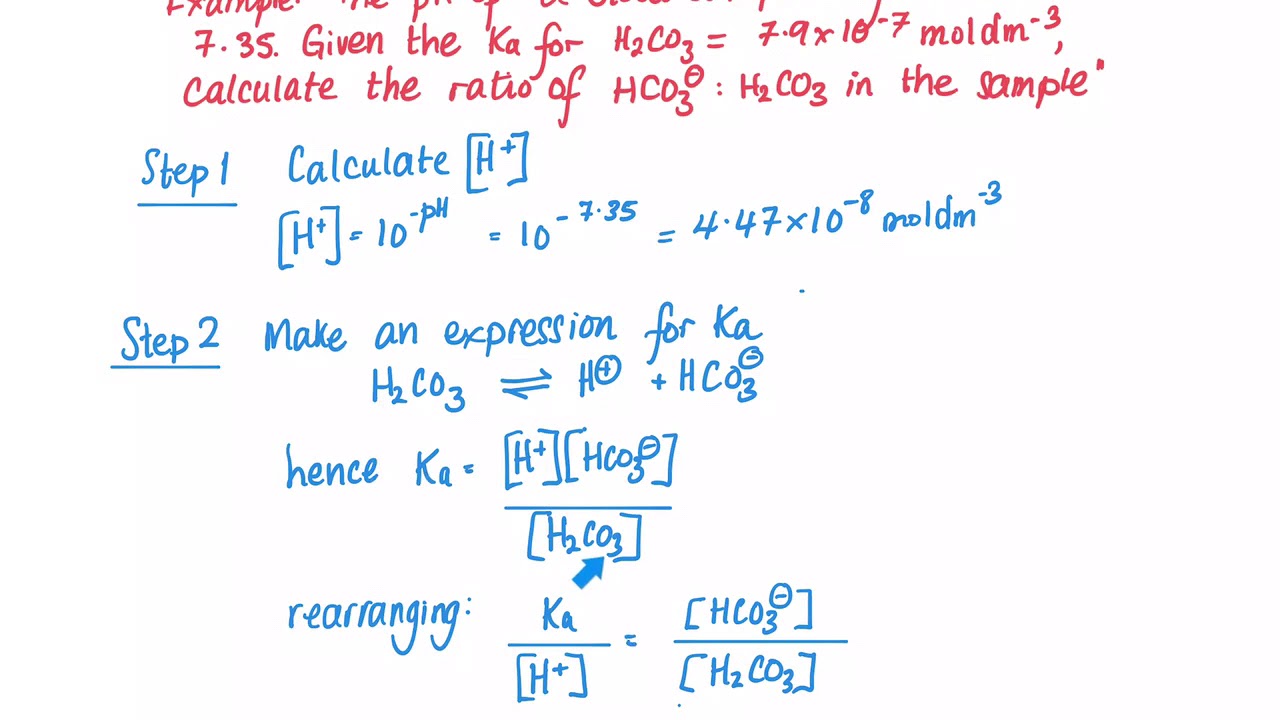
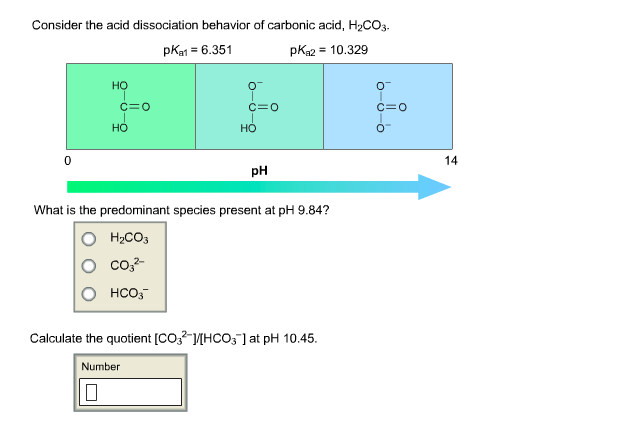
SOLVED: A diprotic acid like carbonic acidhas two pKa values: pKal for the ionization of the first proton; and pKa2 for the ionization ofthe second proton All three related species are present