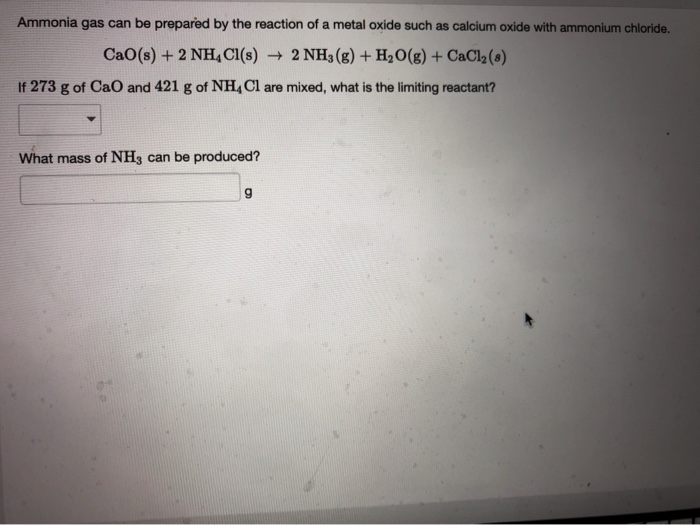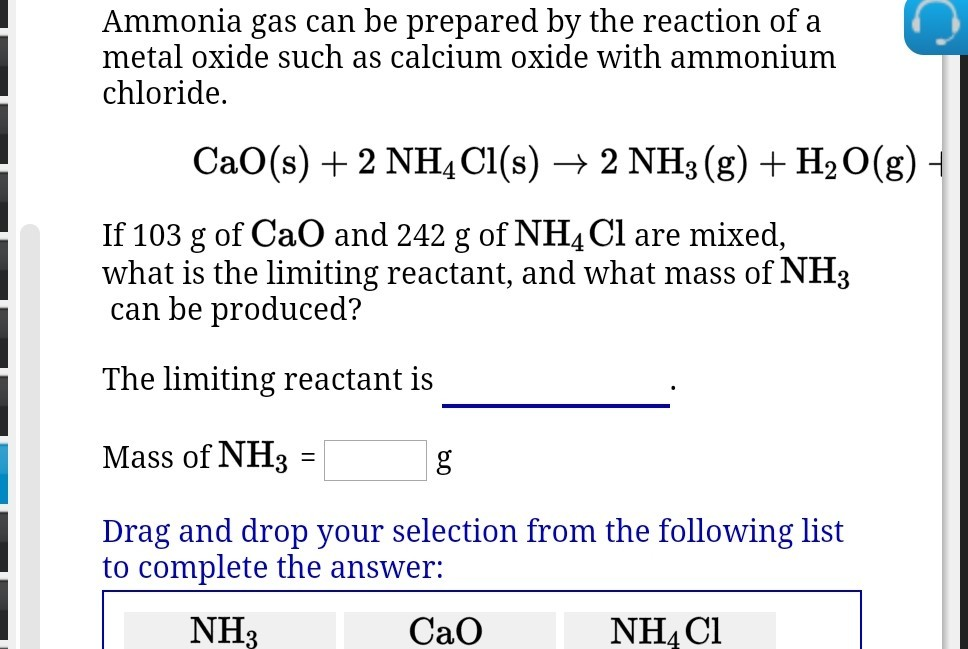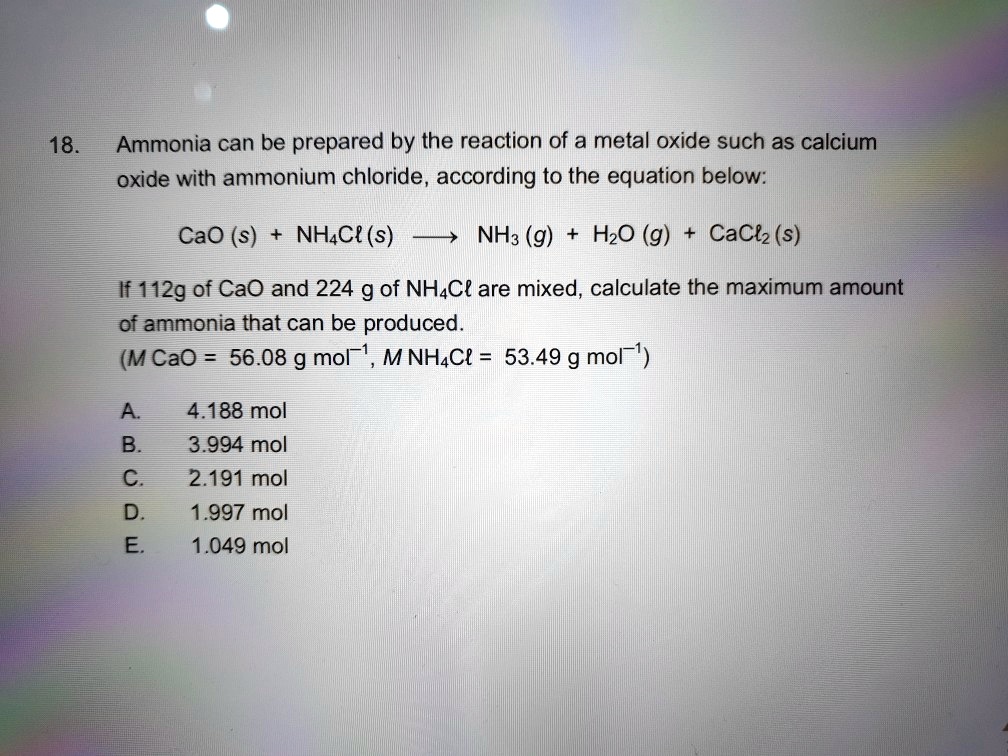
SOLVED: 18 Ammonia can be prepared by the reaction of a metal oxide such as calcium oxide with ammonium chloride , according to the equation below: CaO (s) NHAC? (s) NH: (g)

Nitrogen has a triple bond which is very strong. :N:::N: Only at very high temperatures will it react with oxygen. This occurs in the combustion. - ppt download

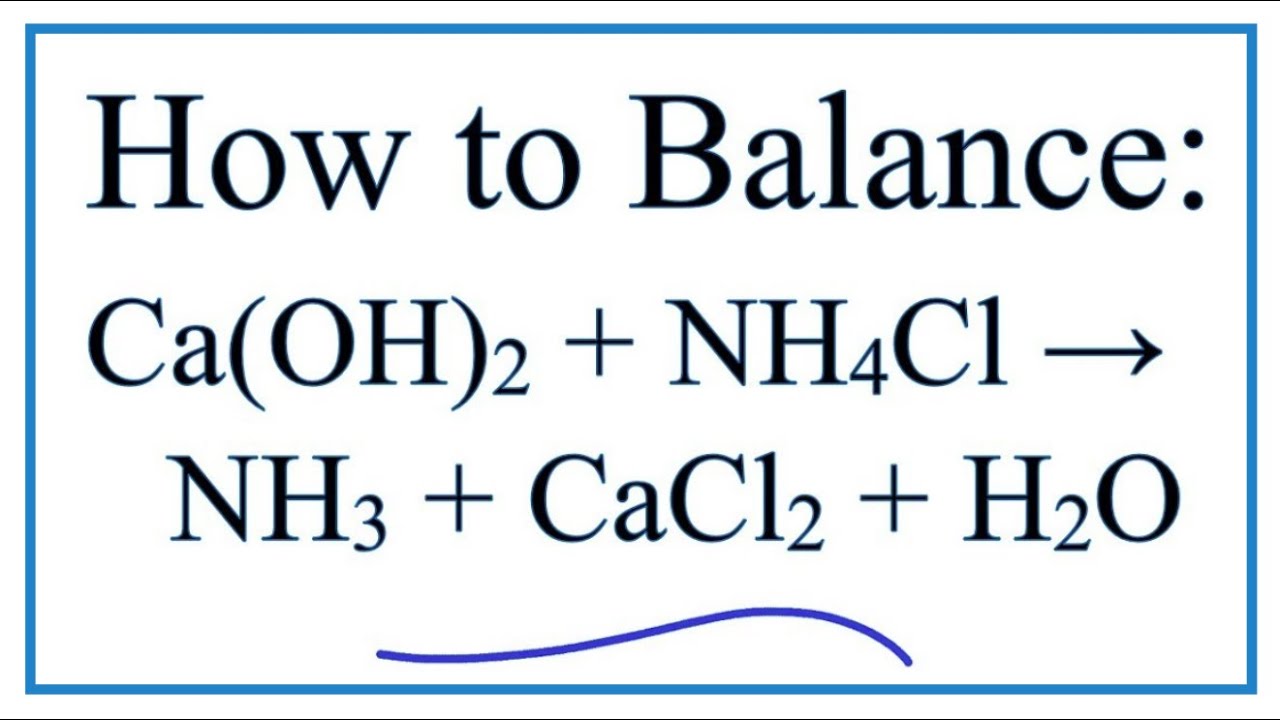
Calcium hydroxide and ammonium chloride react to give ammonia as per equation: Ca(OH)_(2) + 2 NH... - YouTube
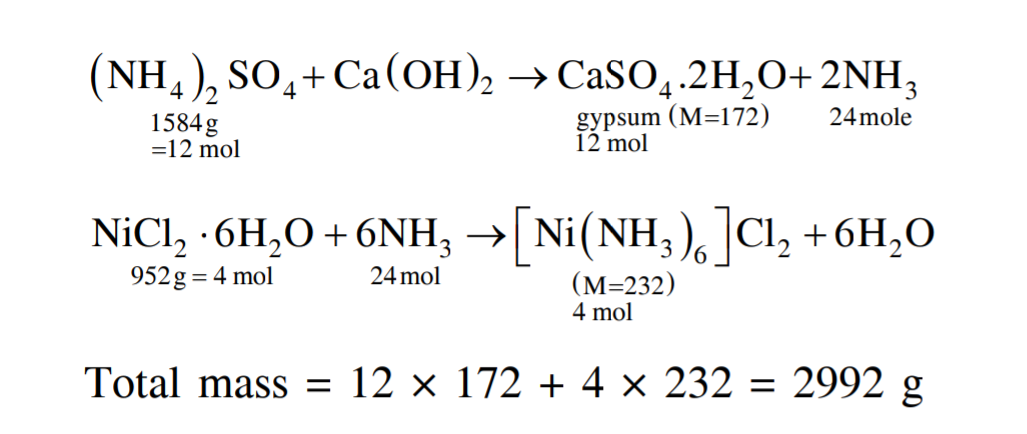
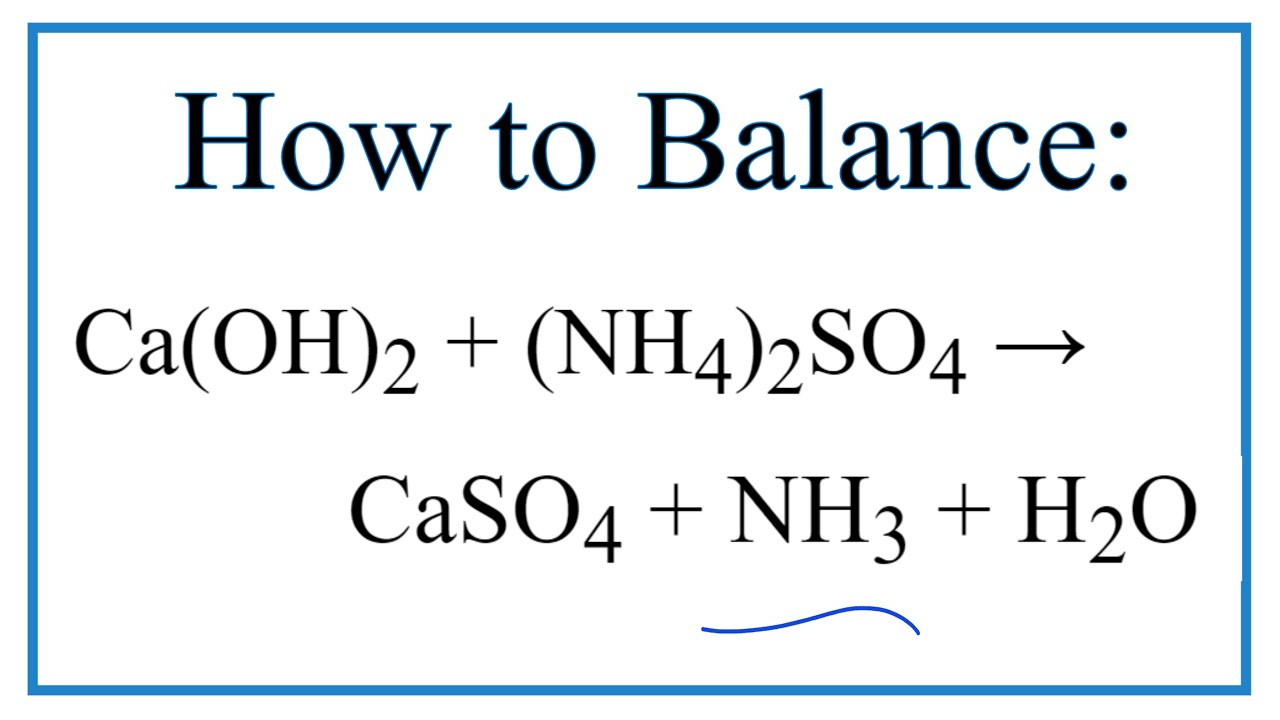
Welcome to Chem Zipper.com......: The ammonia prepared by treating ammonium sulphate with calcium hydroxide is completely used by NiCl2.6H2O to form a stable coordination compound. Assume that both the reactions are 100%

Toward the Mechanistic Understanding of the Additives' Role on Ammonium Nitrate Decomposition: Calcium Carbonate and Calcium Sulfate as Case Studies | ACS Omega

How to Balance the Reaction Between Ammonium Nitrate and Calcium Hydroxide NH4NO3 and Ca(OH)2 - YouTube

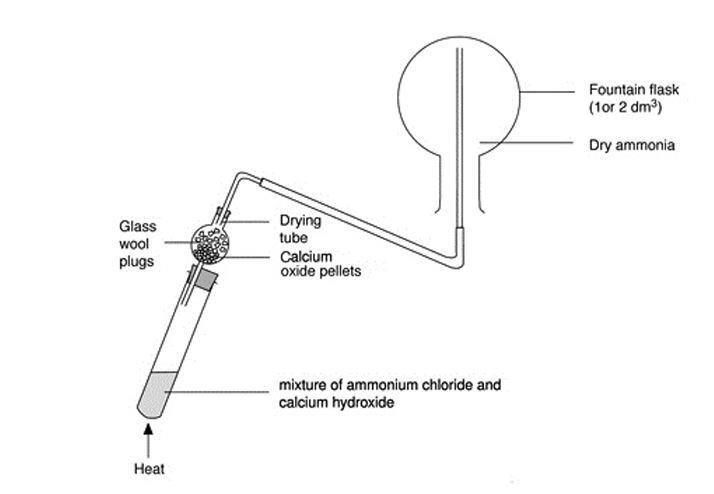
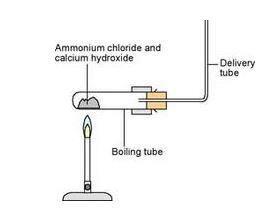
Preparation of Ammonia Gas in Laboratory with the Help of Ammonium Chloride and Calcium Oxide Stock Vector - Illustration of white, formula: 220304379
![Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2021/10/qa-aq-ammonia.jpg?w=986)
Why calcium ions do not form precipitate with aqueous ammonia [online video] – O Level Secondary Chemistry Tuition