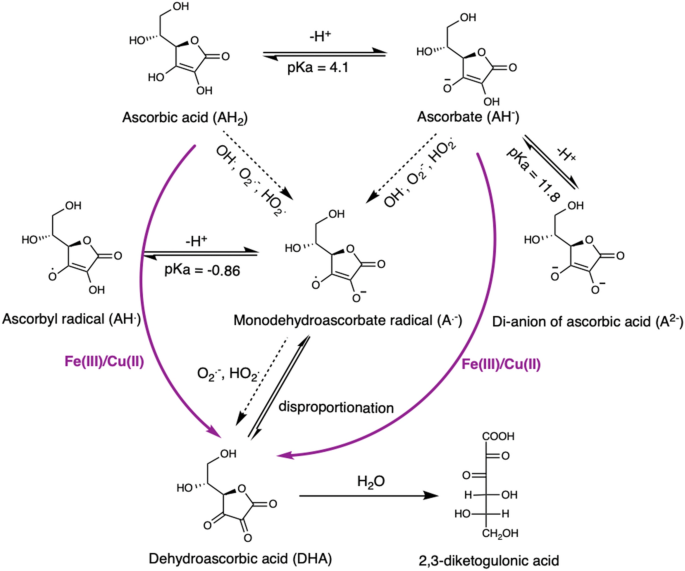
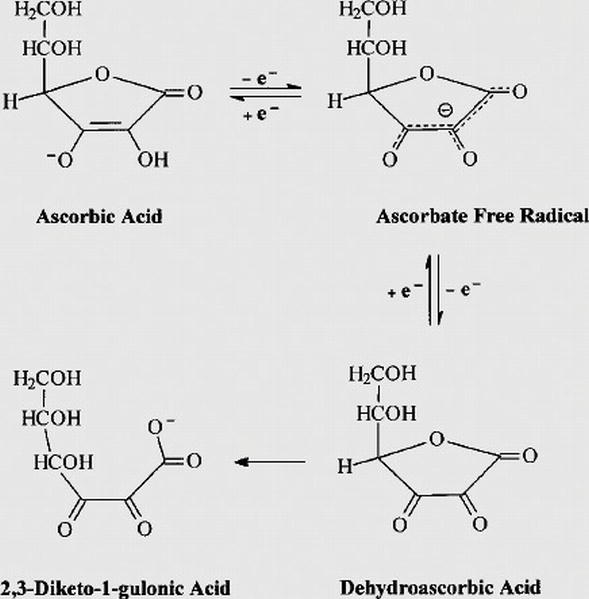
Ascorbate oxidation by iron, copper and reactive oxygen species: review, model development, and derivation of key rate constants | Scientific Reports

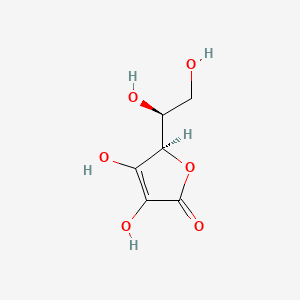
Ascorbic acid (vitamin C) is a diprotic acid, H2C6H6O6 . What is the pH of a 0.10 M solution? The acid ionization constants are Ka1 = 9.0 × 10^-3 and Ka2 = 1.6 × 10^-12 . (log 2 = 0.3, log 3 = 0.48)

Fig. S11 Plot showing the coexistence of ascorbic acid and dissociation... | Download Scientific Diagram

SciELO - Brasil - Thermodynamic Study on the Acid-Base Properties of Antioxidant Compound Ascorbic Acid in Different NaClO<sub>4</sub> Aqueous Ethanol Solutions Thermodynamic Study on the Acid-Base Properties of Antioxidant Compound Ascorbic Acid
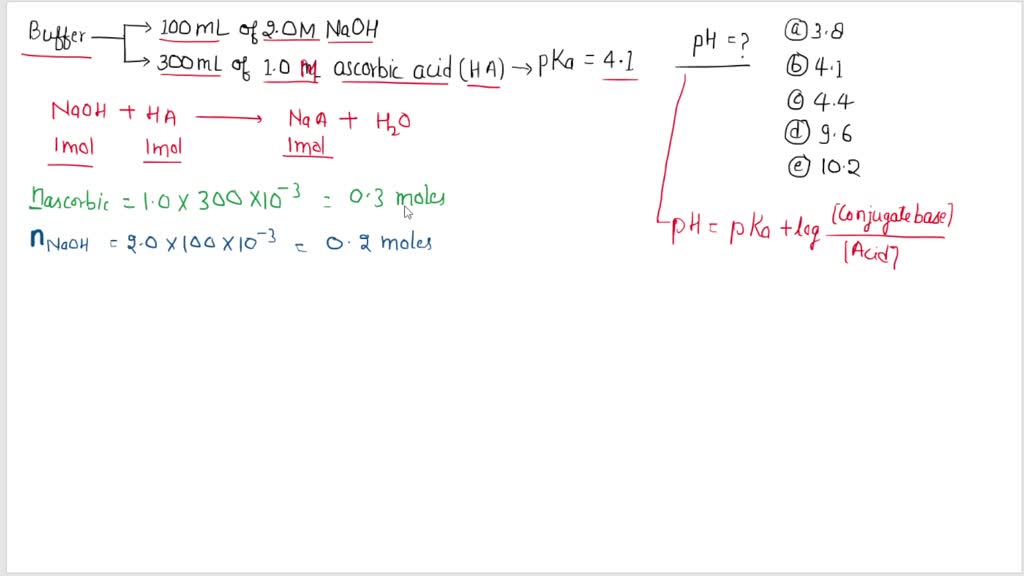
SOLVED: 47. Ascorbic acid (Vitamin C) is a weak acid with Ka = 8.0 x 10-5 ( pKa 4.1) What is the pH ofa buffer solution that is prepared by adding 100 mL
![PDF] Effect of surfactants on the dissociation constants of ascorbic and maleic acids. | Semantic Scholar PDF] Effect of surfactants on the dissociation constants of ascorbic and maleic acids. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6f58dac458fdaa70e1651ac65814b2a7a6f06320/3-Figure1-1.png)
PDF] Effect of surfactants on the dissociation constants of ascorbic and maleic acids. | Semantic Scholar

3-O-ethyl-l-ascorbic acid: Characterisation and investigation of single solvent systems for delivery to the skin - ScienceDirect
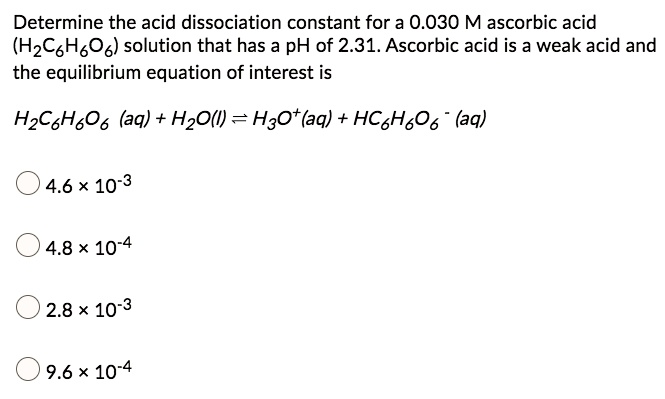
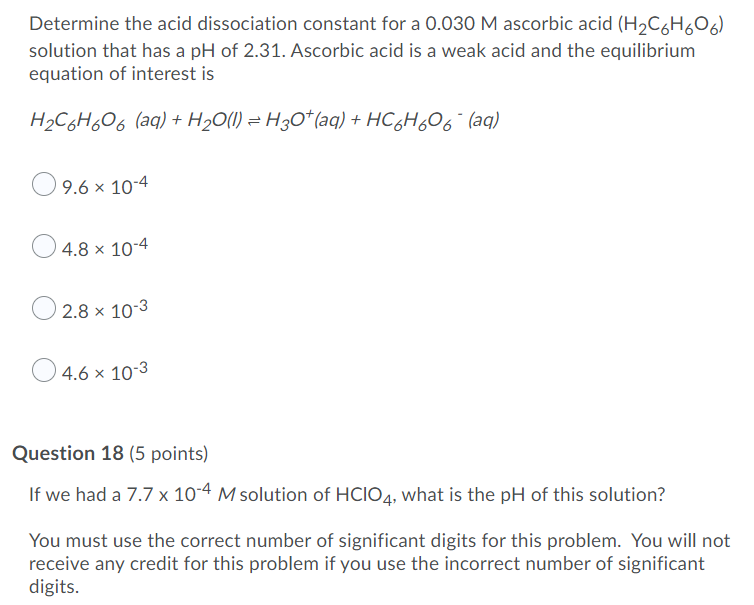
SOLVED: Determine the acid dissociation constant for a 0.030 M ascorbic acid (HzC6H,O6) solution that has a pH of 2.31. Ascorbic acid is a weak acid and the equilibrium equation of interest

1 Acid-Base Equilibria. 2 Solutions of a Weak Acid or Base The simplest acid-base equilibria are those in which a single acid or base solute reacts with. - ppt download
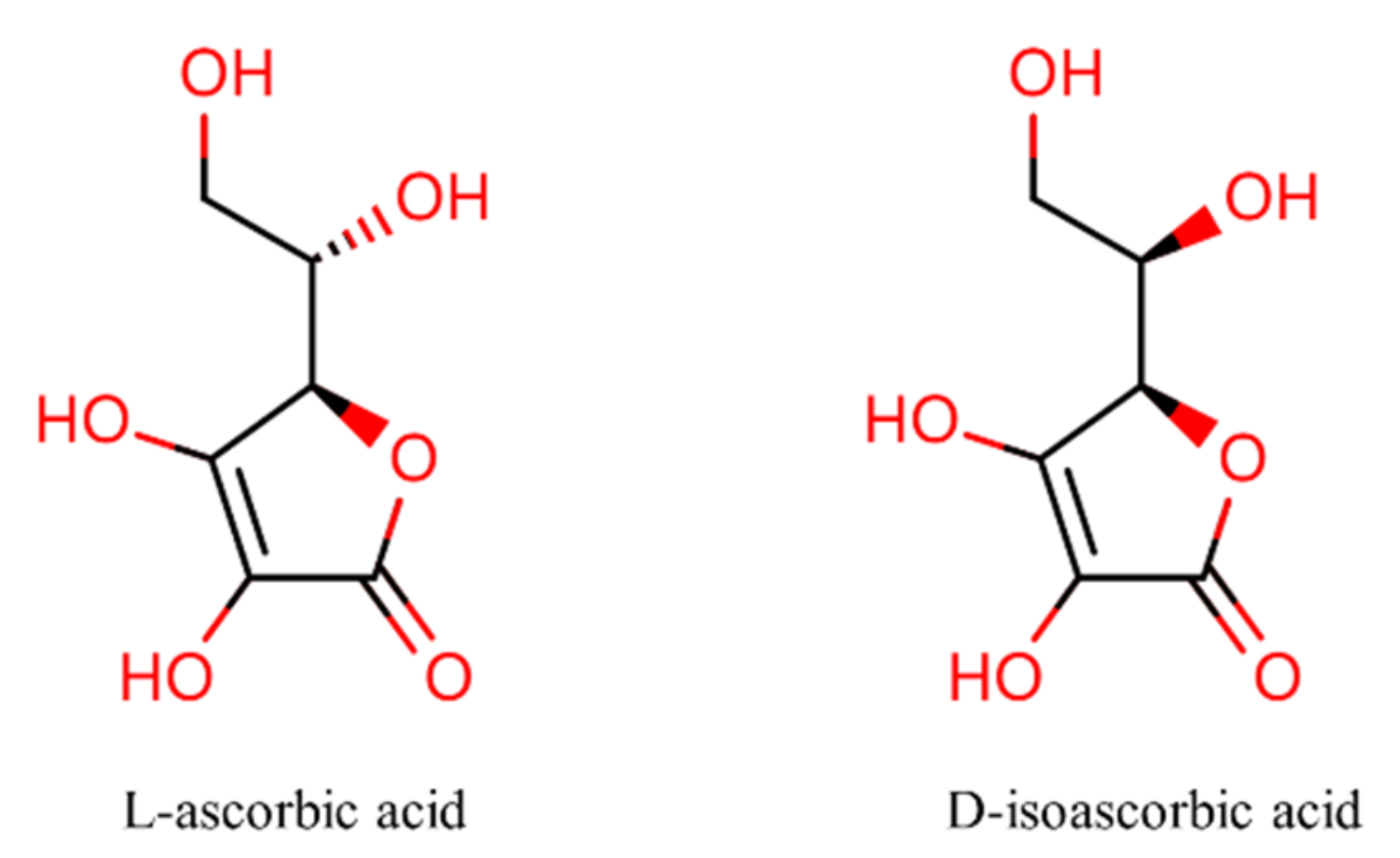
Antioxidants | Free Full-Text | Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology
![PDF] Effect of surfactants on the dissociation constants of ascorbic and maleic acids. | Semantic Scholar PDF] Effect of surfactants on the dissociation constants of ascorbic and maleic acids. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6f58dac458fdaa70e1651ac65814b2a7a6f06320/4-Table3-1.png)
PDF] Effect of surfactants on the dissociation constants of ascorbic and maleic acids. | Semantic Scholar
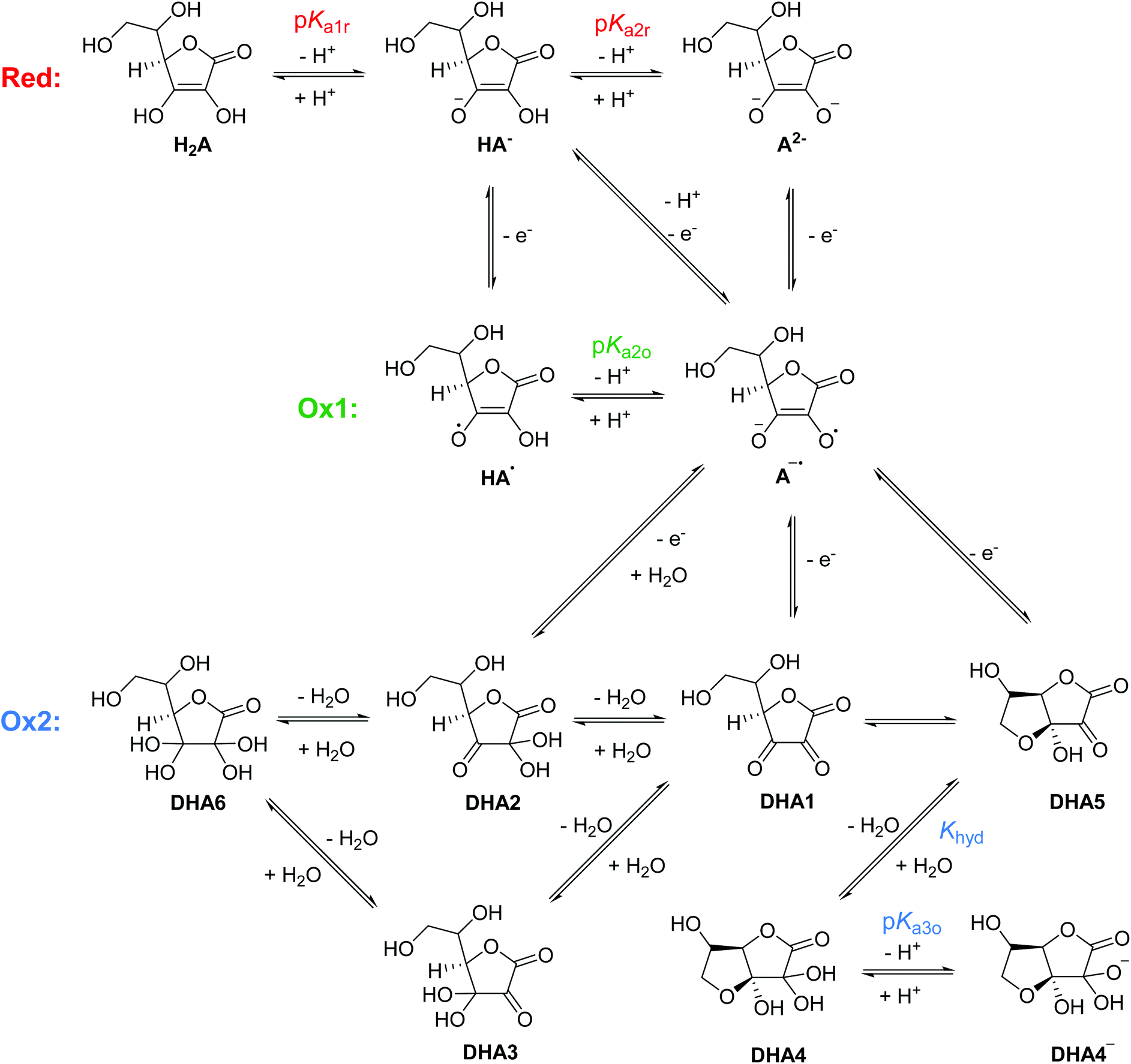
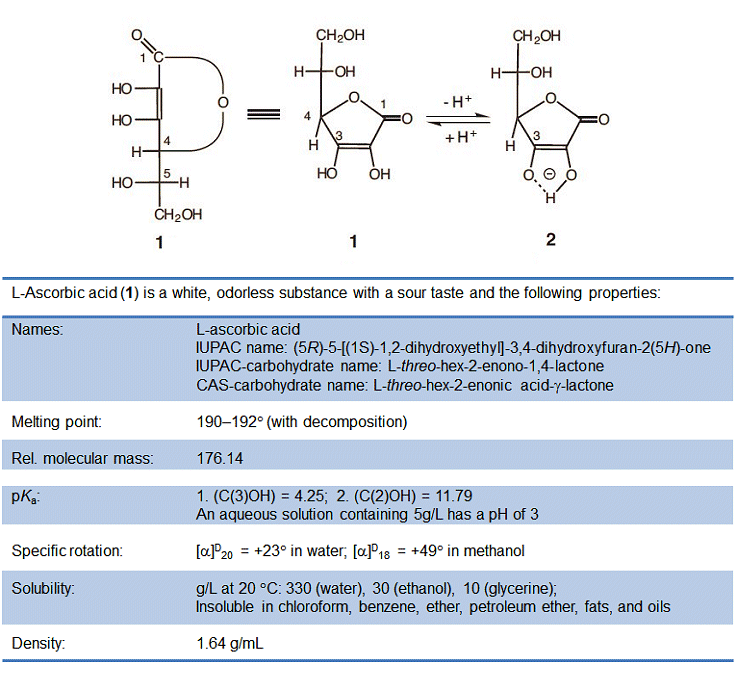
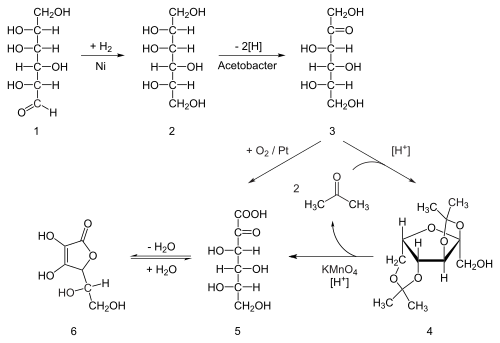




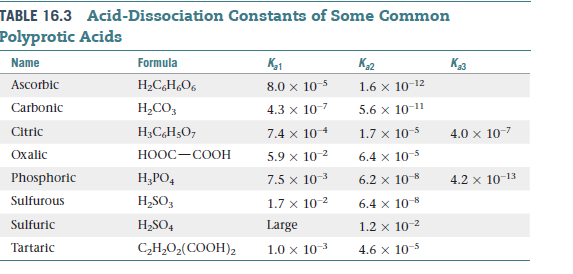


.jpg)

