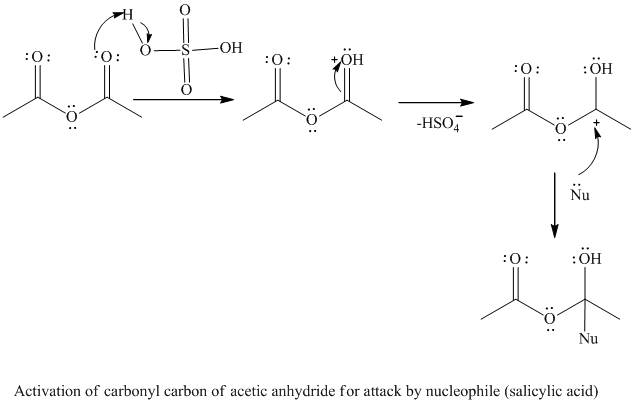
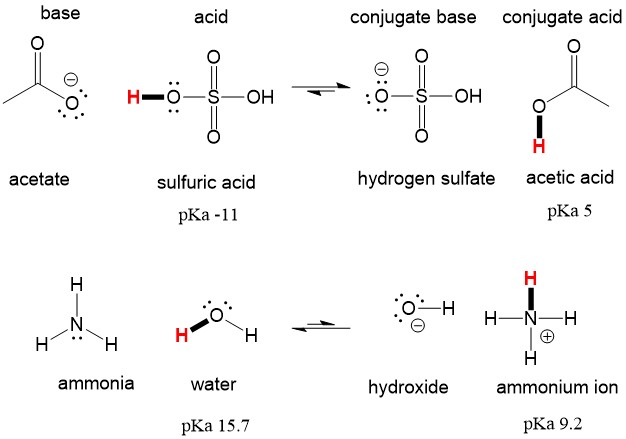
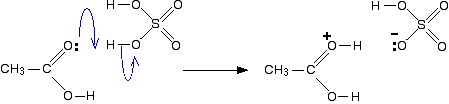
The reaction between salicylic acid and acetic anhydride, which is catalyzed by sulfuric acid and produces aspirin and acetic acid is given. Write an equation for a reaction that might form salicylic

Sulfuric Acid, Hydrochloric Acid, Nitric Acid, Glacial Acetic Acid, Formaldehyde, Formic Acid Supplier

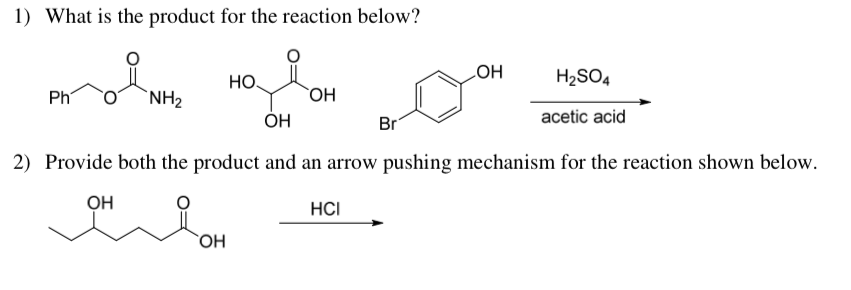
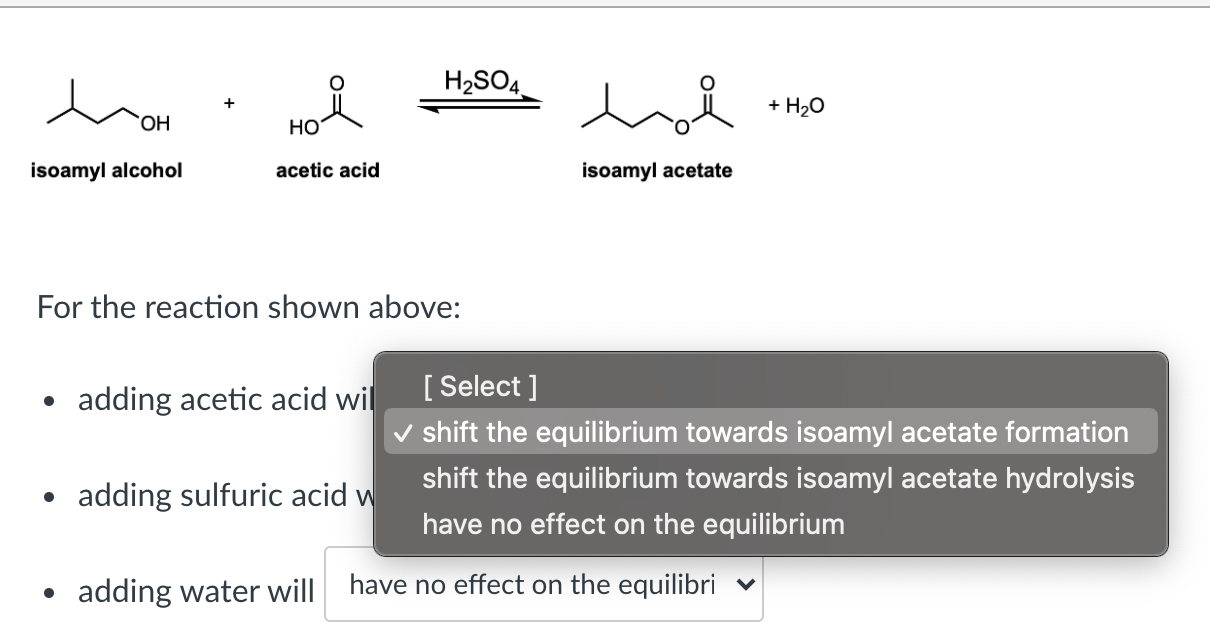
What product is obtained from the reaction of sulfuric acid, acetic acid and 1-pentanol? | Homework.Study.com
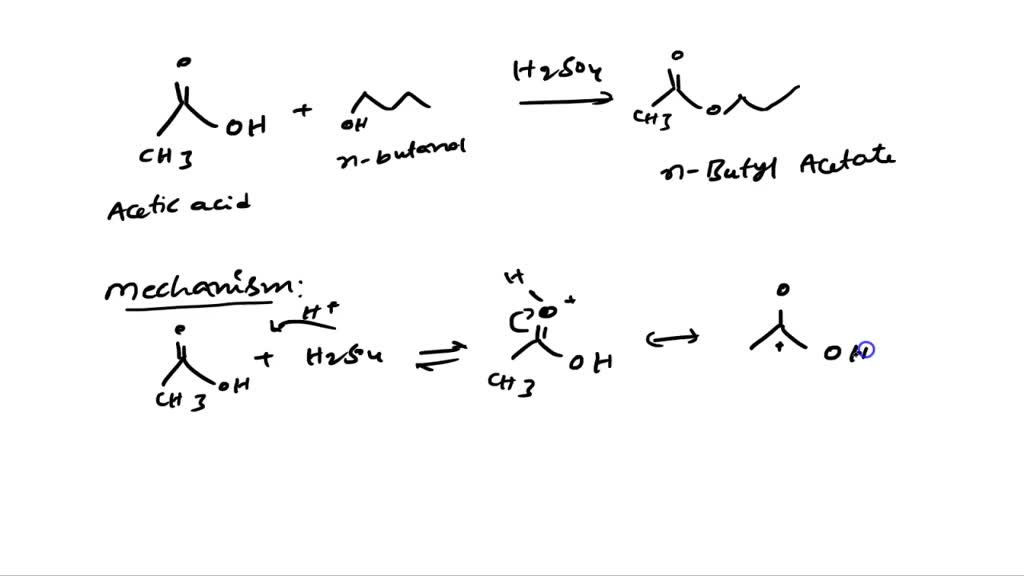
SOLVED: Reaction Type: Overall Reaction of 1-butanol and acetic acid to form n-butyl acetate using sulfuric acid as a catalyst: Hand-written Fischer Esterification Mechanism of 1-butanol and acetic acid to form n-butyl

Sulfuric Acid, Hydrochloric Acid, Nitric Acid, Glacial Acetic Acid, Formaldehyde, Formic Acid Supplier

Write a detailed mechanism for a. the Fischer esterification of acetic acid with ethanol in the presence of sulfuric acid and b. the reaction of acetyl chloride with ethanol. Explain which reagent

Write a reaction mechanism for Fischer esterification of primary alcohol with acetic acid using sulfuric acid as a catalyst. Include lone electron pairs, electron flow and arrows. | Homework.Study.com