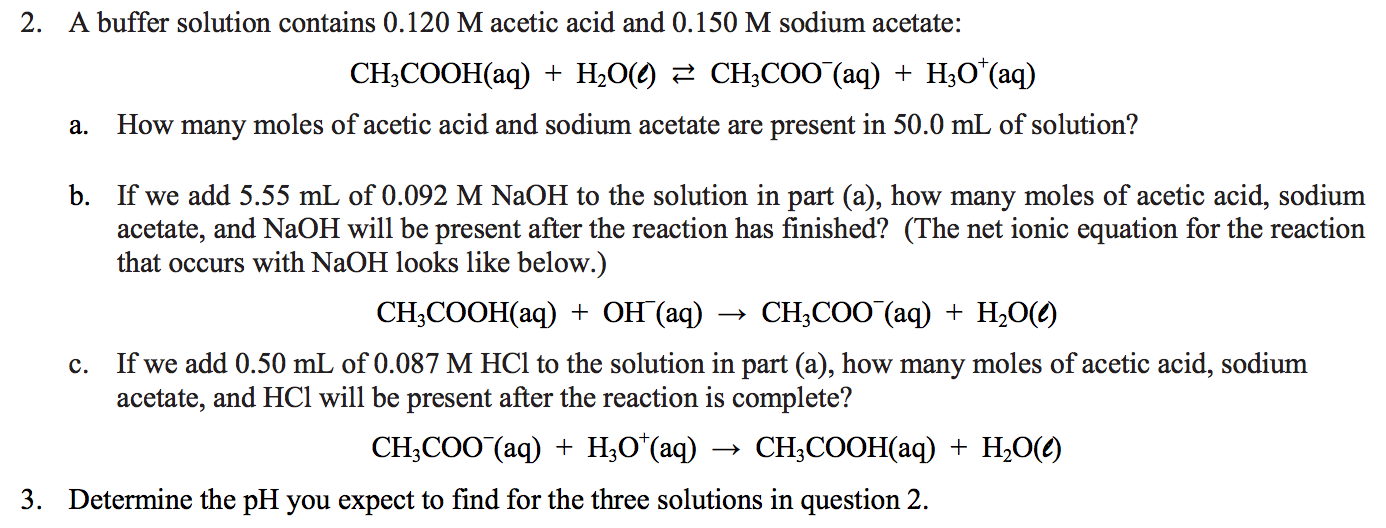
1 Chapter 10 Acids and Bases 10.9 Buffers. 2 When an acid or base is added to water, the pH changes drastically. A buffer solution resists a change in. - ppt download

SOLVED: buffer solution is made from acetic acid, HCzH;Oz, and sodium acetate, NaCzH;Oz: Suppose small amount of hydrochloric What is the net ionic equation for the reaction that occurs when acid is
![SOLVED: [10]4. The pH of a sodium acetate-acetic acid buffer is 4.5. Calculate the ratio of [CH3COOH]/[CH3COO-]. [Ka of CH3COOH is 1.8x10-5] SOLVED: [10]4. The pH of a sodium acetate-acetic acid buffer is 4.5. Calculate the ratio of [CH3COOH]/[CH3COO-]. [Ka of CH3COOH is 1.8x10-5]](https://cdn.numerade.com/ask_previews/03ad68ba-cab0-4fc6-ad7e-62059a06cfe3_large.jpg)
SOLVED: [10]4. The pH of a sodium acetate-acetic acid buffer is 4.5. Calculate the ratio of [CH3COOH]/[CH3COO-]. [Ka of CH3COOH is 1.8x10-5]

OneClass: A buffer contains significant amounts of acetic acid and sodium acetate. Write an equation ...
![The pH of an acetic acid + sodium acetate buffer is given by pH = pK(a) + log . "[Salt]"/"[Acid]" " where " K(a) of acetic acid = 1.8 xx 10^(-5) If [ The pH of an acetic acid + sodium acetate buffer is given by pH = pK(a) + log . "[Salt]"/"[Acid]" " where " K(a) of acetic acid = 1.8 xx 10^(-5) If [](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/52405160_web.png)
The pH of an acetic acid + sodium acetate buffer is given by pH = pK(a) + log . "[Salt]"/"[Acid]" " where " K(a) of acetic acid = 1.8 xx 10^(-5) If [

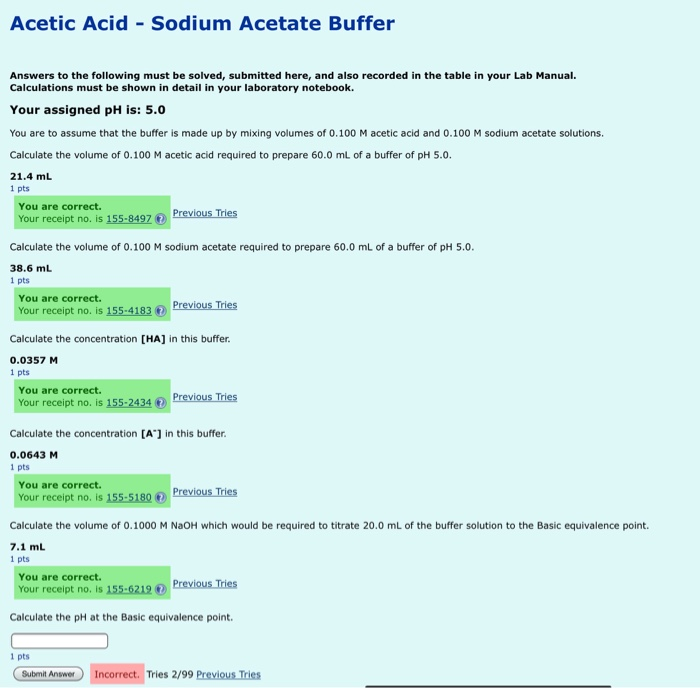
SOLVED: Part 4: Making an acetate buffer Reagents and glassware: SOmL 0.OM acetic acid Calculated mass of sodium acetate (which may be sodium acetate trihydrate) this mass will be less than IOg
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/dDlCNVZnUE9URzQ=/sd/)
Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]
For preparing a buffer of pH 6 by mixing sodium acetate and acetic acid the ratio of the concentration of salt and acid should be ( Ka=10^ 5)
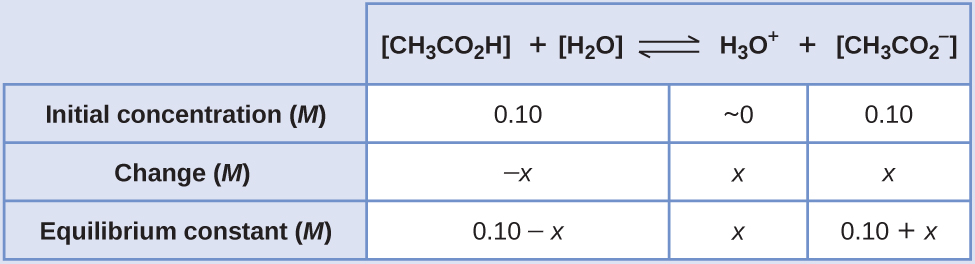
Compare 1 L of acetate buffer solution (0.50 mol of acetic acid and 0.50 mol sodium acetate) to 1 L of HCl solution Similarities

SCH 4 U 1. What are buffers? Buffers are mixtures of conjugate acid- base pairs that allow a solution to resist changes in pH when acids and/or bases. - ppt download