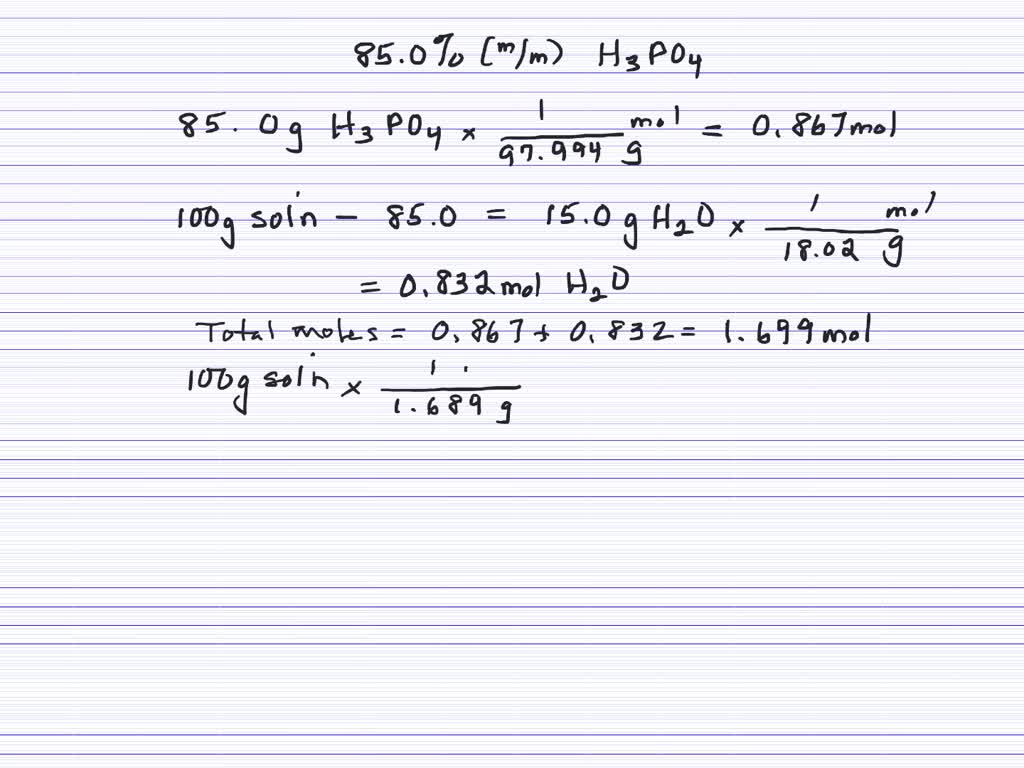
SOLVED:A bottle of phosphoric acid is labeled “ 85.0 % H3 PO4 by mass; density =1.689 g / cm^3 . " Calculate the molarity, molality and mole fraction of the phosphoric acid in solution.
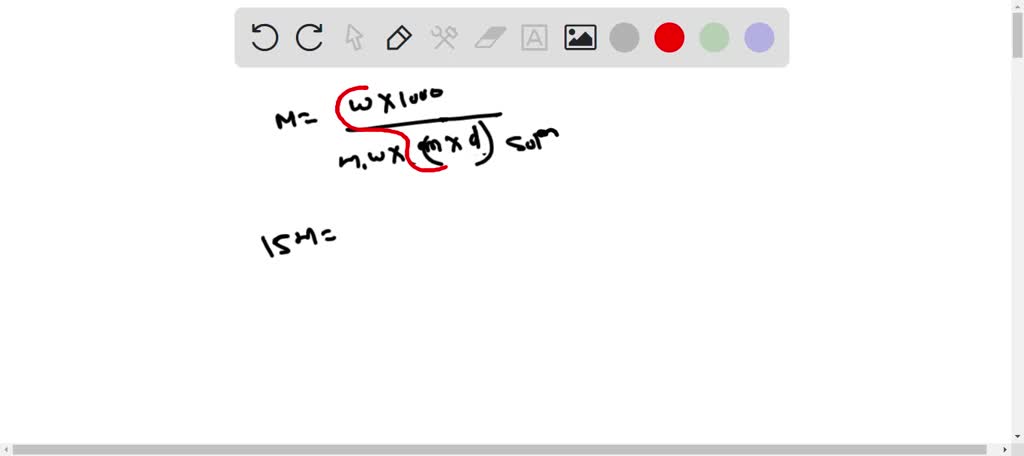
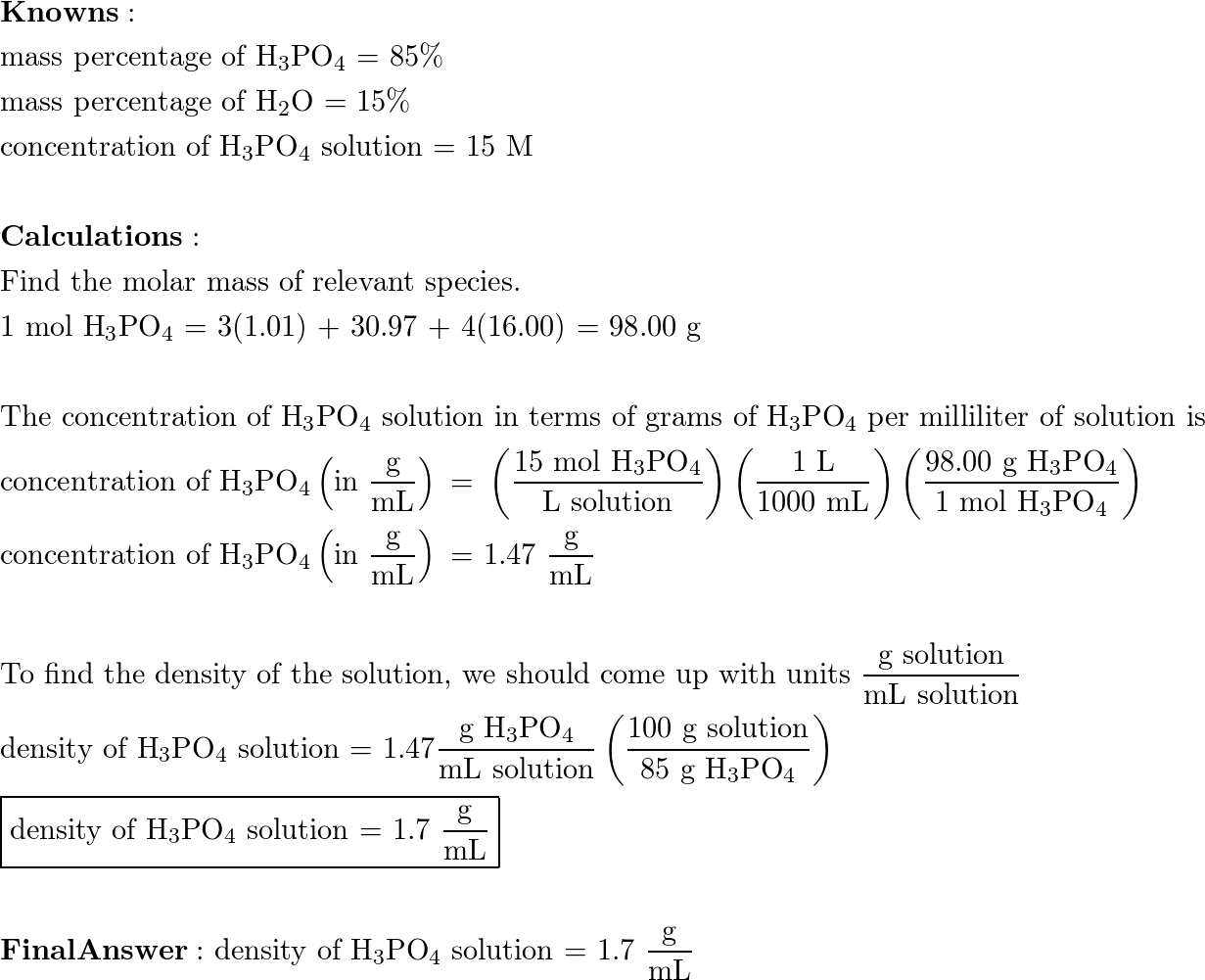
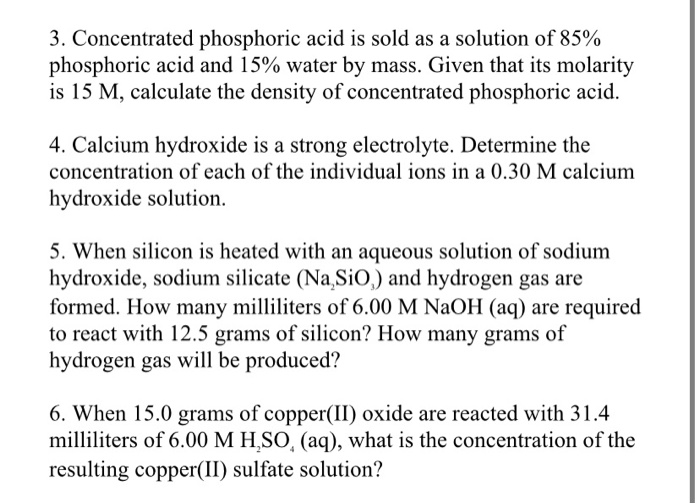
SOLVED: Concentrated phosphoric acid is sold as a solution of 85 % phosphoric acid and 15 % water by mass. Given that its molarity is 15 M, calculate the density of concentrated phosphoric acid.

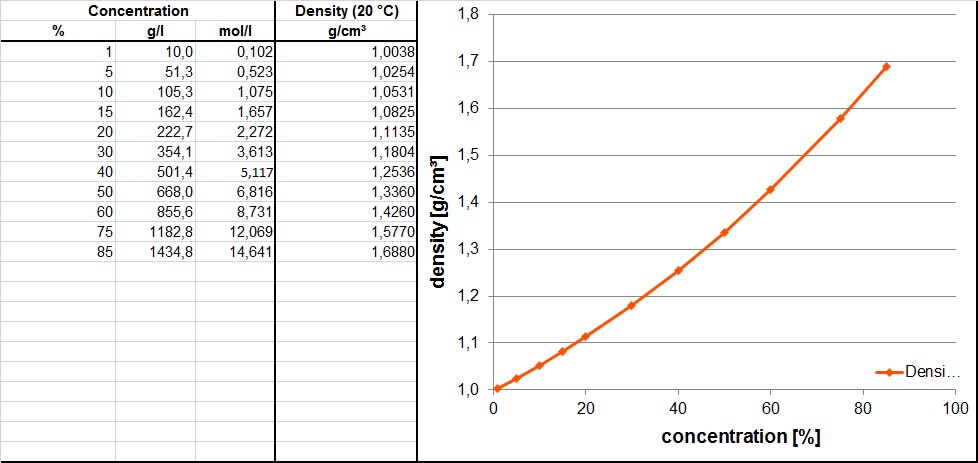
The density of 85% Phosphoric acid is 1.70 g cm^-3 . What is the volume of a solution that contains 17 gm Phosphoric acid?

SOLVED: A solution of 85.0% by mass phosphoric acid has a density of 1.685 g/mL. What is the molarity of the H3PO4 solution?
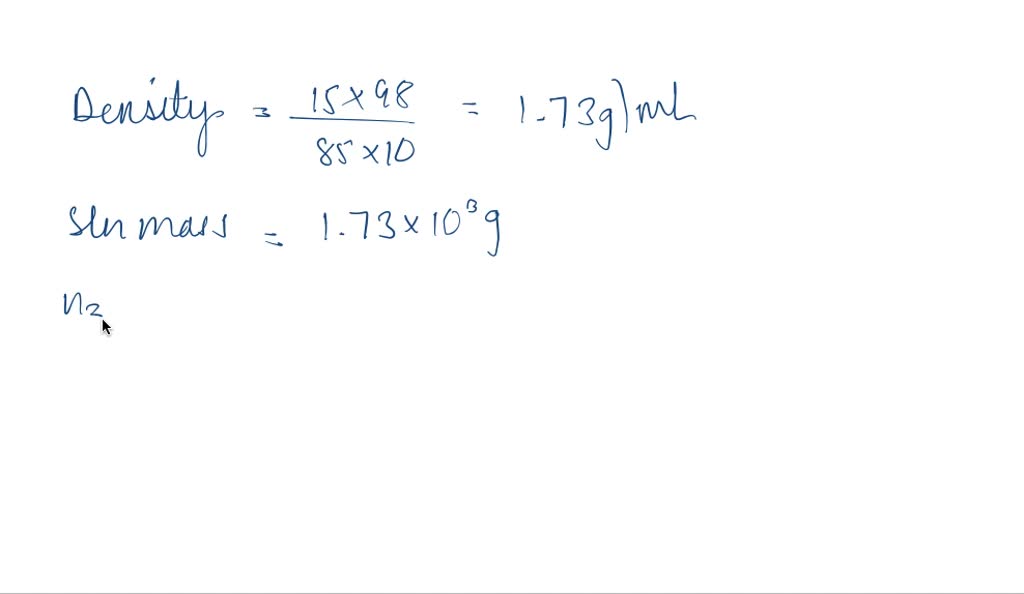
SOLVED: Q94. What is the molarity of a phosphoric acid solution if the solution is 85% by mass H3PO4 and has a density of 1.7 g/mL? [Hint: the solute is H3PO4 and

Selection of stainless steels for handling phosphoric acid (H3PO4) – British Stainless Steel Association
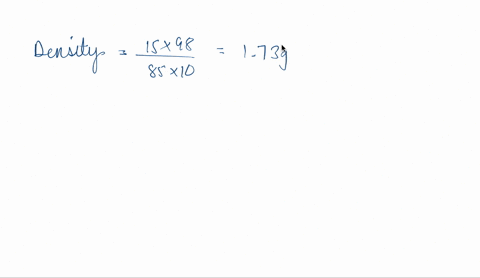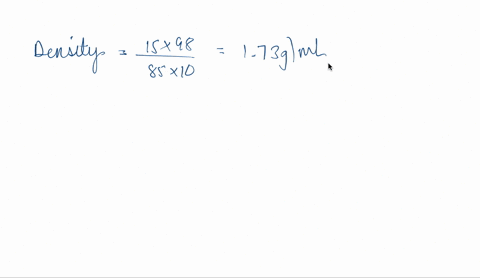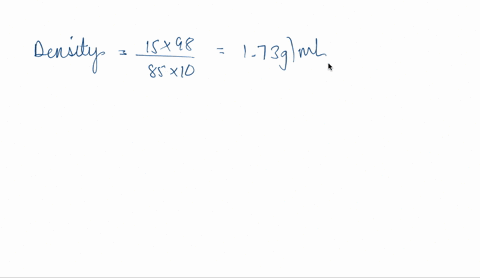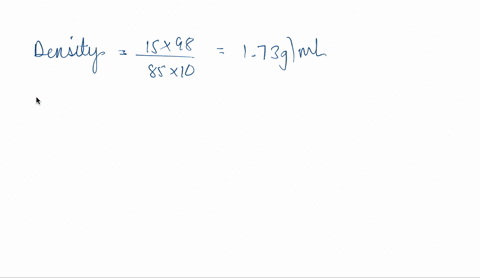
SOLVED: phosphoric acid, 85% purity density =1.88 g/ml) AND the Calculate the volume of commercial of water needed to prepare SOOmL of 0.5 M phosphoric acid solution. (MW: 98g/mol) volume (1 pt)

SOLVED: A concentrated phosphoric acid solution is 85.5% H3PO4 by mass and has a density of 1.69 g/mL at 25°C. What is the molarity of H3PO4? What is the mole fraction of

A concentrated phosphoric acid solution is 85.doc - A concentrated phosphoric acid solution is 85.5% H3PO4 by mass and has a density of 1.69 g/mL at | Course Hero